Acetic Acid’s Ka: The Silent Power Behind One of Nature’s Most Versatile Acids
Acetic Acid’s Ka: The Silent Power Behind One of Nature’s Most Versatile Acids
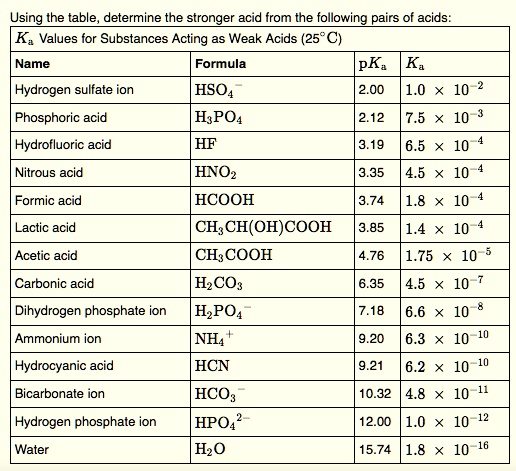
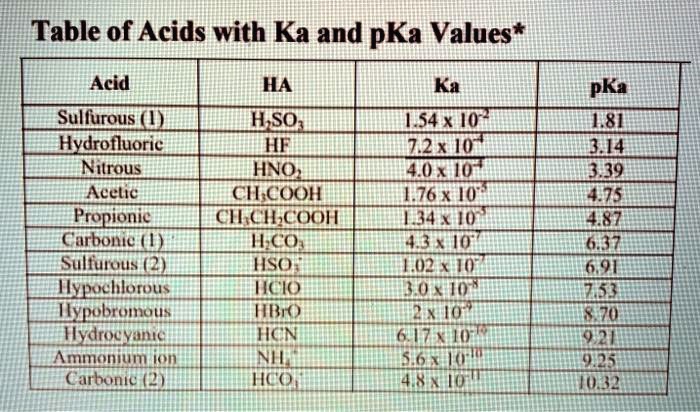
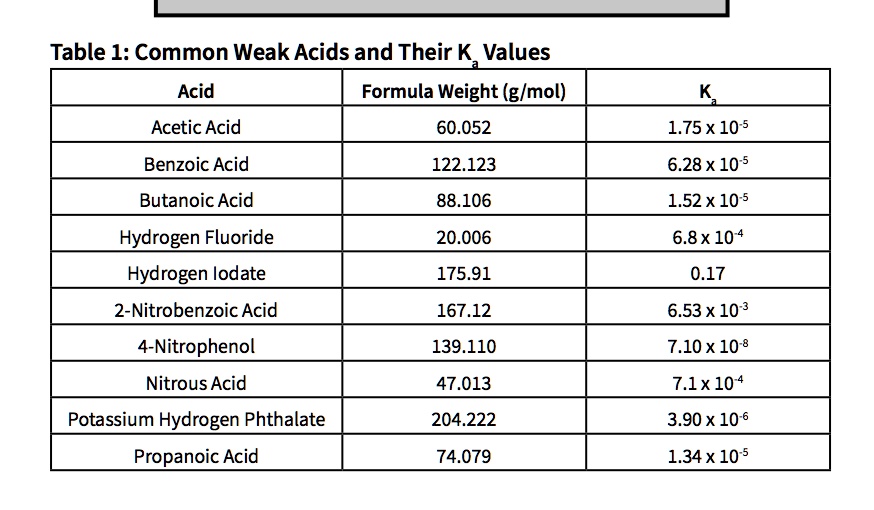
Acetic acid, a simple yet profoundly influential compound, hinges on a precise chemical property—its acidity constant, Ka—to govern its reactivity, stability, and widespread applications across industry, biology, and consumer products. With a Ka value around 1.8 × 10⁻⁵ at 25°C, this weak acid exemplifies how subtle variations in molecular behavior underpin real-world utility. From tempering sauces to powering green solvents, acetic acid’s Ka dictates not just its pH, but its entire functional role in chemistry and beyond.
The Chemistry of Acetic Acid and Its Ka
Acetic acid (CH₃COOH) is a carboxylic acid characterized by a carboxyl functional group—an acidic proton attached to a carbonyl carbon flanked by a hydroxyl.
Its dissociation in water follows a reversible process: CH₃COOH ⇌ H⁺ + CH₃COO⁻. The equilibrium between these species is quantified by its acid dissociation constant, Ka, defined as the product of the concentration of hydrogen ions and acetate ions divided by the concentration of undissociated acetic acid. A Ka of 1.8 × 10⁻⁵ reflects its moderate strength—neither fully dissociated like strong acids nor so inert as weak dust.
This intermediate behavior makes acetic acid uniquely reliable.
"Its Ka establishes a predictable dissociation pattern, enabling precise control in chemical synthesis and buffering systems," notes Dr. Elena Torres, an organic chemist at the Institute for Applied Chemical Sciences. "This balance allows acetic acid to act as both a catalyst and a stabilizing agent, depending on the reaction conditions." The log Ka (pKa ≈ 4.74) further fine-tunes its performance: a lower log Ka indicates stronger acidity relative to water’s autoionization, making acetic acid sufficiently acidic to influence proton transfer yet gentle enough for delicate biological environments.
Industrial Applications Shaped by Acetic Acid’s Ka
The Ka value is not merely a laboratory curiosity—it directly shapes how acetic acid is deployed across manufacturing, agriculture, and science.
In vinegar production, the controlled fermentation of ethanol yields acetic acid, where the Ka governs acidity levels critical to flavor and preservation. A pH around 2.4, derived from this equilibrium, inhibits microbial growth while delivering the tang essential to culinary traditions worldwide.
Beyond food, acetic acid’s Ka enables eco-friendly innovation. In green chemistry, it serves as a safer alternative to volatile solvents like dichloromethane.
Its moderate acidity allows efficient esterification reactions for biodegradable plastics and coatings, where precise catalytic activity—dictated by Ka—is essential for yield and purity. In pharmaceuticals, acetic acid's Ka aids in drug formulation: buffering systems ensure stability and bioavailability, crucial for dosage accuracy. Cosmetics, textiles, and cleaning agents all rely on this balance—every application a testament to the acid’s measurable, predictable power.
Biological Relevance: Acetic Acid’s Role in Life’s Chemistry
Acetic acid’s Ka is equally significant within living systems.
In human metabolism, acetate—derived from acetyl-CoA—enters the tricarboxylic acid (TCA) cycle, with its Ka influencing proton release and energy production. In the rhumen of cattle, microbial fermentation generates acetic acid, contributing to beef’s distinctive properties. The pH stability conferred by its Ka ensures enzymes function optimally, maintaining metabolic flux.
Even in pathology, Ka reveals insight.
In diabetic ketoacidosis, elevated ketone bodies alter systemic acidity; understanding acetic acid’s equilibrium helps clinicians monitor and correct pH imbalances. “The Ka isn’t just a number—it’s a bridge between molecular behavior and organismal health,” explains Dr. Julia Mendez, biochemist at the

Related Post

Demon Slayer Ringtones: Download Your Favourite Anime Sounds — Immerse in the Battle Without Leaving Your Phone

Capital Of The Holland: Amsterdam’s Enduring Legacy as the Heart of Dutch Governance and Culture

Juegos No Bloqueados

All Movies Hub 4U: Your Ultimate Destination for Film Enthusiasts