Are Lipids Hydrophobic or Hydrophilic? The Definitive Guide to Nature’s Secret Fat Structure
Are Lipids Hydrophobic or Hydrophilic? The Definitive Guide to Nature’s Secret Fat Structure
Beneath the microscopic surface of every living cell lies a dynamic layer of lipids—molecules so fundamental to life yet often misunderstood in their molecular behavior. The question of whether lipids are hydrophobic or hydrophilic is not just a matter of chemistry—it’s a cornerstone of biological function. Truly, lipids are predominantly hydrophobic, but their structural complexity reveals a nuanced interaction with water and polar environments.
This article unpacks the lipid’s dual identity, explores how molecular architecture dictates function, and reveals why this property underpins everything from cellular membranes to energy storage.
At their core, lipids are a diverse group of biomolecules characterized primarily by their insolubility in water and solubility in organic solvents—classic hallmarks of hydrophobicity. The hydrocarbon tails of most lipids, composed mainly of long chains of carbon and hydrogen atoms, resist interaction with polar water molecules.
“Lipids tend to avoid water because they are nonpolar, and water molecules strongly form hydrogen bonds with each other,” explains Dr. Elena Torres, a biophysicist at the Institute for Molecular Biology. “This avoidance drives lipids to cluster, minimizing contact with water and forming structures like biological membranes.” In contrast, the polar head groups—such as phosphate, glycerol, or cholesterol—introduce limited hydrophilic character, creating a finely balanced interface where lipids interact selectively with aqueous environments.
The Molecular Blueprint: Hydrophobic Tails and Hydrophilic Heads
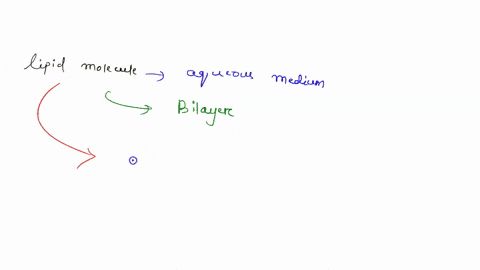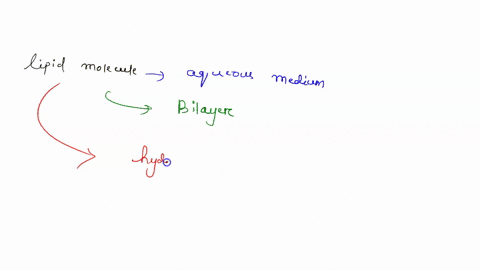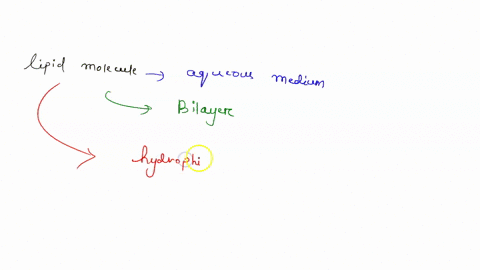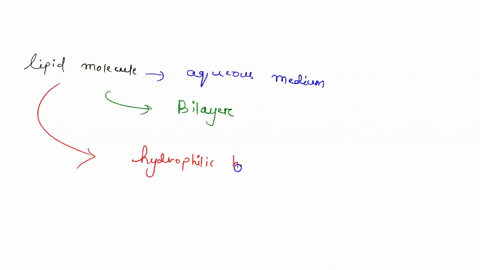
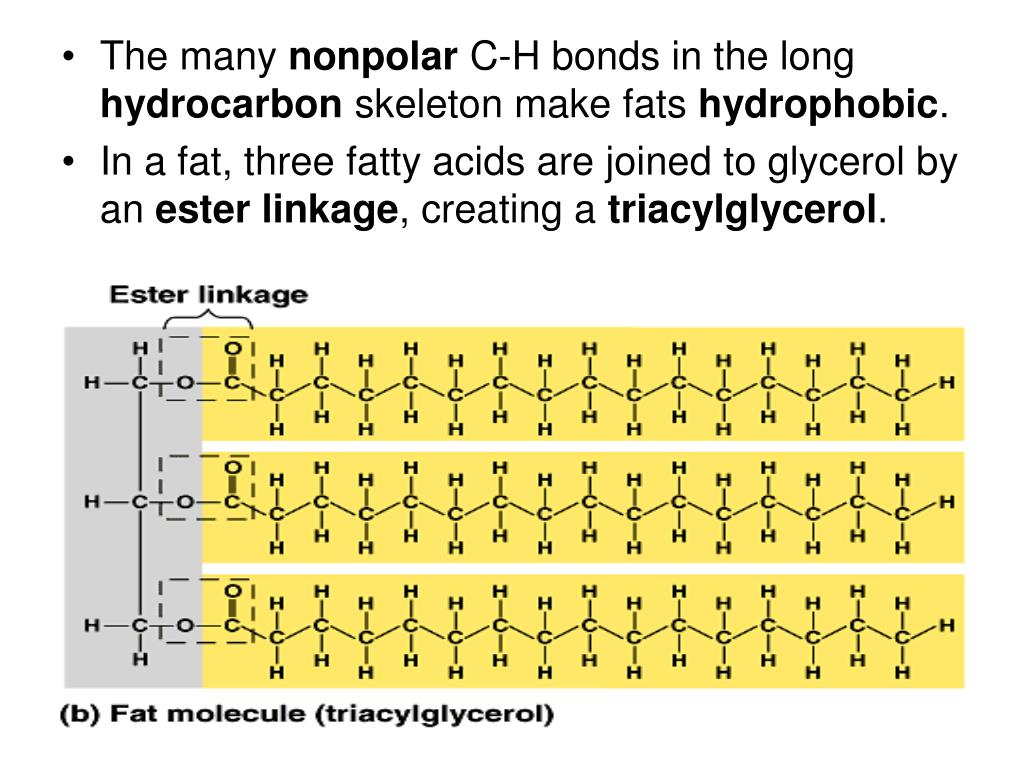
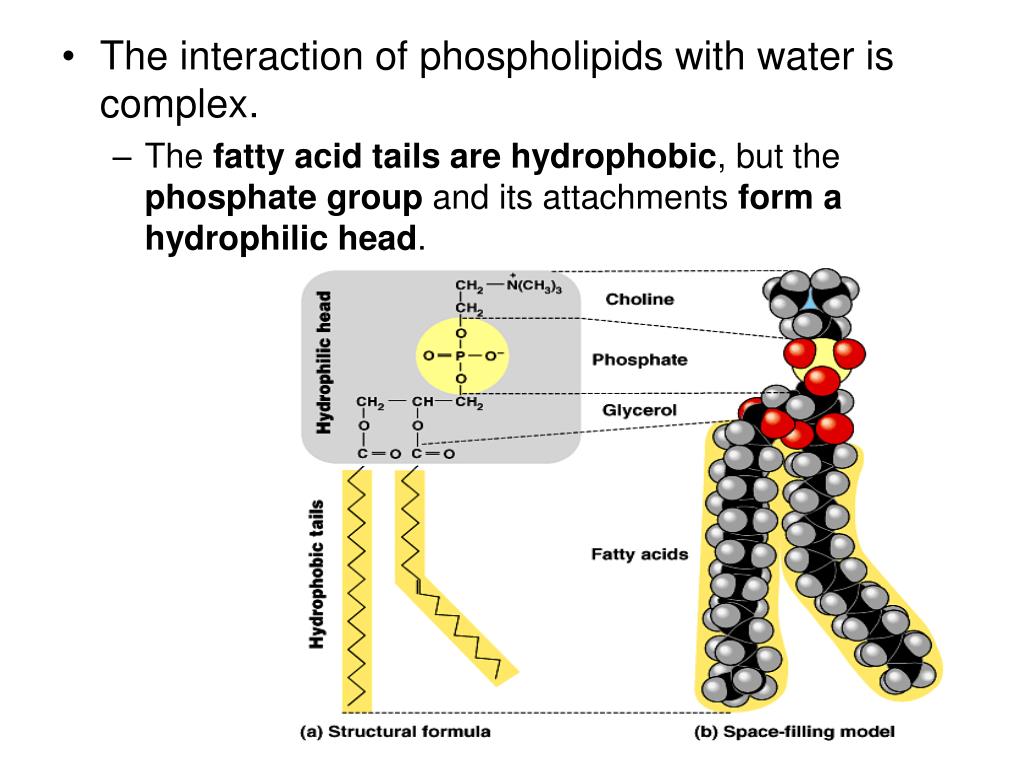
Understanding lipid hydrophobicity begins with dissecting their molecular structure. Lipids are not a single compound but a class of biomolecules with key structural features that determine their behavior: - **Hydrophobic Tails**: Typically long chains of hydrocarbons—fatty acids or glycerol backbones—with no charge and minimal polarity. These alkyl chains naturally reject water due to strong entropic penalties when Claire surrounded by polar molecules.- **Hydrophilic Head Groups**: Polar functional groups such as phosphate (-PO₄), hydroxyl (-OH), or steroid rings, which engage in hydrogen bonding and electrostatic interactions with water. These groups provide limited water compatibility but do not override the dominant nonpolar nature. - **Amphipathic Nature**: Many biological lipids—like phospholipids and sphingolipids—are amphipathic: they possess both hydrophobic and hydrophilic regions.
This dual affinity allows them to form bilayers, the structural basis of cell membranes. <
Lipid Classifications: Variability Within Hydrophobicity
Not all lipids behave the same—classifications reveal the spectrum of hydrophobic expression: - Fatty Acids: The simplest hydrophobic building blocks, consisting of a carboxyl head and a hydrocarbon chain. Highly soluble in nonpolar solvents but insoluble in water. - Phospholipids: The primary components of cellular membranes.These amphipathic molecules form spontaneous bilayers in water, with tails packed inland and heads facing outward—creating a selective, dynamic barrier. - Sphingolipids: Structurally related to phospholipids but built on a sphingosine backbone instead of glycerol. They reinforce membrane integrity and participate in signaling.
- Steroid Lipids: Including cholesterol, these molecules lack a hydrophilic head. Cholesterol modulates membrane fluidity by inserting between phospholipids, balancing rigidity and permeability. - Waxes and Triglycerides: Triacylglycerols store energy with highly hydrophobic tails and minimal hydrophilic resistance; their low solubility makes them ideal for protective coatings.
Each class leverages hydrophobicity in tailored ways—from membrane formation to energy reserves—yet all rely on the principle that lipids avoid polar environments unless strategically amphipathic.
Biological Functions Shaped by Lipid Hydrophobicity
The dominant hydrophobic nature of lipids enables critical biological roles: - Cell Membrane Architecture: The phospholipid bilayer’s hydrophobic interior shields proteins from polar environments while allowing selective permeability. “This structure forms the fundamental plasma membrane,” notes Dr.Torres. “Without lipid hydrophobicity, cells couldn’t maintain internal organization.” - Energy Storage: Triglycerides store more than twice the energy per gram compared to carbohydrates, owing to dense hydrocarbon tails that resist water-driven metabolic breakdown. - Insulation and Protection: Subcutaneous fat layer acts as a hydrophobic barrier—trapping heat and shielding organs—while the waxy cuticles of plants repel pathogens and water loss.
- Signaling Pathways: Lipid-derived messengers like steroid hormones (e.g., cortisol, estrogen) traverse cell membranes effortlessly due to their lipid solubility. Conversely, in aqueous biological settings—such as blood plasma or the cytoplasm—lipids must be sequestered within amphipathic assemblies to prevent disruptive aggregation or interference with aqueous processes.
Hydrophilic Moments: When Lipids Embrace Water
Though lipids are largely hydrophobic, certain molecular modifications and structural contexts introduce functional hydrophilicity: - Lysophospholipids: Created when a fatty acid chain is missing from a phospholipid, leaving one polar head and one tail—enhancing solubility.- Glycolipids: Sugars link to glycolipids, adding hydrophilic carbohydrate heads that aid cell recognition without compromising hydrophobic tails. - Membrane Microdomains: Lipids cluster into rafts rich in cholesterol and sphingolipids, where local ordering increases fluidity and selective permeability. These rare exceptions highlight that while the lipid class is defined by hydrophobic dominance, hydrophilic moments strategically emerge to fine-tune function—not structural identity.
Future Implications: Lipid Hydrophobicity in Medicine and Technology
Understanding lipid behavior is accelerating breakthroughs in medicine and materials science. Liposome-based drug delivery systems exploit hydrophobic pockets within lipid bilayers to encapsulate and target therapies, improving efficacy and reducing side effects. Nanotechnology uses hydrophobic-lipid nanoparticles to mimic cell membranes for biosensing and tissue engineering.In metabolic research, disruptions in lipid hydrophobicity contribute to diseases like obesity, diabetes, and neurodegeneration. Advanced lipidomics now map cellular lipid landscapes to diagnose dysfunctions before symptoms appear, transforming early intervention. <

Related Post

Unlocking Bond Duration: The Precision Measure That Shapes Fixed Income Investment Strategy

Adult Children of Emotionally Immature Parents: Unveiling the Hidden Wounds and Pathways to Healing

WTF Games: Where Absurd Humor Collides with Gameplay Genius

Create Hatsune Miku Vocals: The Definitive Guide to Crafting Iconic Vocal Artistry

