Cerebral Amyloid Angiopathy: The Silent Brain Threat Behind Silent Strokes
Cerebral Amyloid Angiopathy: The Silent Brain Threat Behind Silent Strokes
Cerebral Amyloid Angiopathy (CAA) represents a complex and often underestimated neurodegenerative condition in which abnormal amyloid protein deposits accumulate in brain blood vessel walls—triggering inflammation, vascular fragility, and a heightened risk of cerebral hemorrhage. Often overshadowed by Alzheimer’s disease, CAA is increasingly recognized as a primary cause of lobar hemorrhagic strokes in older adults. In reviews by UWorld, the pathology of CAA bridges vascular and neurodegenerative medicine, revealing how amyloid-driven vascular damage silently undermines brain health over decades.
This condition affects approximately 5–10% of individuals over 80 worldwide, yet remains a diagnostic challenge due to overlapping symptoms with other age-related brain disorders. Understanding CAA—its mechanisms, clinical footprint, and evolving management—is critical for timely intervention and preventing devastating neurological outcomes.
The Pathophysiology: How Amyloid Disrupts Brain Vessels
Cerebral Amyloid Angiopathy arises predominantly from deposits of amyloid-β (Aβ) protein—best known as the primary component of Alzheimer’s plaques—but in CAA, it accumulates within the mural layers of cortical and leptomeningeal arteries. Unlike protective clearance mechanisms in the brain, erroneous breakdown of Aβ leads to chronic vascular deposition, weakening vessel walls and promoting microfibrillar rupture.
The leading theory posits that these deposits trigger a cascade: amyloid infiltration incites immune infiltration, activation of matrix metalloproteinases (MMPs), and progressive inflammation, all contributing to vessel fragility. Over time, this structural compromise increases susceptibility to spontaneous hemorrhage—often in the cerebral cortex or base—without the typical warning signs of systemic vascular disease.Although amyloid accumulation is ubiquitous in aging, only a subset of exposed individuals develop clinically evident CAA, suggesting complex genetic and environmental modifiers remain to be fully elucidated.
Clinical Manifestations: From Silent Hemorrhages to Catastrophic Strokes
One of CAA’s most insidious features is its ability to cause neurological deterioration without overt vascular symptoms. While acute symptomatic hemorrhage—often lobar and deep—occurs in 15–20% of cases, a substantial number present with subtler, insidious cognitive decline resembling early dementia or subtle focal deficits such as lava flow hemispheric dysfunction.
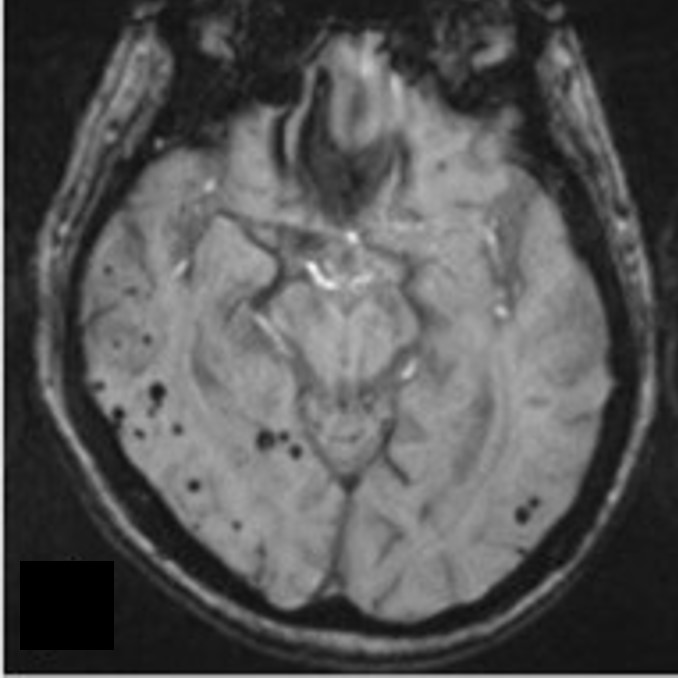
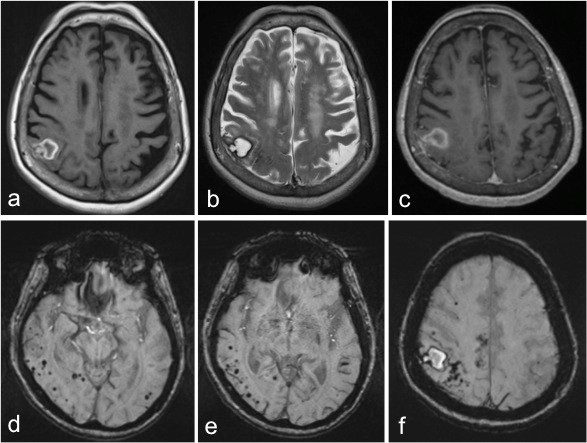
These "silent strokes" accumulate over years, contributing to vascular cognitive impairment without an obvious hemorrhagic event. Paradoxically, CAA may coexist with Alzheimer’s pathology, complicating both diagnosis and treatment. Features such as lobar hemorrhages on brain imaging, cognitive subtleties, and MRI signs like cortical superficial siderosis (visible on susceptibility-weighted imaging) are telltale clues.
The UWorld anatomical review emphasizes that early recognition hinges on interpreting these neuroimaging hallmarks in the context of age and clinical presentation.
Diagnosis: Navigating the Gray Zones of CAA Confirmation
Diagnosing Cerebral Amyloid Angiopathy presents significant challenges due to symptom overlap with Alzheimer’s disease, small vessel disease, and other forms of brain vascular injury. Traditional imaging such as CT or standard MRI may miss microhemorrhages or cortical patterns critical for CAA suspicion. Modern neuroimaging—particularly susceptibility-weighted imaging (SWI) and fluid-attenuated inversion recovery (FLAIR) combined with gradient-recall inversion (`GRINF2` sequences)—has dramatically improved detection accuracy, revealing microbleeds and superficial siderosis with high sensitivity.
Pathological gold standard remains postmortem autopsy showing cortical amyloid deposition, but live diagnostic tools are advancing rapidly. Serum and CSF biomarkers under investigation include glymphatic signaling molecules and specific Aβ isoforms, aiming to detect early amyloid vascular deposition before irreversible damage occurs. Yet, absence of a single definitive test means CAA is often inferred from clinical and imaging convergence—making multidisciplinary evaluation essential.
Risk Factors and Demographics: Who Is Most Vulnerable?
While CAA affects primarily older adults, particularly those over 75, emerging data highlight a complex interplay of genetic and environmental risk factors.
Age remains the strongest determinant: increases in CAA prevalence parallel advancing years, peaking in individuals over 85. Genetic influences are increasingly clear: the apolipoprotein E (APOE) ε4 allele, a well-established Alzheimer’s risk factor, independently elevates susceptibility to CAA—especially the amyloid-positive but cognitively inconspicuous variant. Recent genome-wide association studies (GWAS) have identified variants in genes linked to amyloid metabolism and vascular integrity, such as *CLU*, *CR1*, and *ABCG1*, reinforcing the dual vascular-neurodegenerative nature of CAA.
While age and genetics dominate, emerging research suggests vascular risk factors—hypertension, diabetes, and atrial fibrillation—may accelerate CAA progression, though chronic CAA often outpaces traditional cardiovascular risk contributions in clinical vulnerability.
Management Challenges and Therapeutic Frontiers
Currently, no curative treatment exists for Cerebral Amyloid Angiopathy, management focuses on reducing hemorrhage risk and supporting cognitive function. Anticoagulation and antiplatelet therapy remain controversial due to the high bleeding risk from cortical amyloid, guiding many clinicians toward a conservative, individualized approach. Small, randomized trials of amyloid-targeting immunotherapies—promising in Alzheimer’s—are ongoing but complicated by concerns that aggressive amyloid clearance in CAA vessels might provoke immune-mediated inflammation or hemorrhage.
Lifestyle interventions such as blood pressure control, optimized glycemic management, and cardiovascular risk mitigation play a critical secondary role in slowing progression. Novel research explores modifying amyloid clearance pathways, targeting inflammatory cascades with novel biologics, and leveraging precision medicine based on genetic profiles. As understanding deepens, a shift toward early risk stratification and personalized monitoring may emerge as transformative strategies.
The Silent Epidemic: CAA’s Hidden Toll on Global Brain Health
Cerebral Amyloid Angiopathy stands at the crossroads of vascular pathology and degenerative brain disease, responsible for a growing proportion of late-life hemorrhagic strokes and cognitive decline worldwide.
Its silent progression—often hidden within the silent damage of amyloid-laden vessels—challenges both clinicians and researchers to sharpen diagnostic acumen and refine risk-based screening. As the global population ages, CAA demands urgent attention not only as a clinical diagnosis but as a collision of neurodegeneration and vascular fragility shaping millions of aging brains. Advances in imaging, biomarkers, and targeted therapies offer cautious optimism, yet current limitations underscore the need for alert healthcare systems and proactive research.
Without timely recognition, CAA continues to strike without warning—reminding us that behind every stroke lies a story of silent amyloid damage unfolding in the deep corridors of the brain.