Cystic Fibrosis Karyotype: Unlocking the Genetic Blueprint of a Complex Genetic Disorder
Cystic Fibrosis Karyotype: Unlocking the Genetic Blueprint of a Complex Genetic Disorder
Cystic fibrosis, a life-altering autosomal recessive disease, stems from mutations in the CFTR gene, but the broader karyotype reveals critical insights into genetic inheritance, diagnostic precision, and long-term disease management. While clinical symptoms dominate patient care, the underlying karyotype—comprising chromosomal structure and CFTR gene integrity—powers advancements in genetic testing, carrier screening, and targeted therapies. Understanding the CysticFibrosisKaryotype is essential to decoding not only diagnosis but also the hereditary patterns that shape countless families worldwide.
The karyotype in cystic fibrosis extends beyond the single-gene defect; it encompasses chromosomal architecture and the specific CFTR mutations present. The CFTR gene, located on chromosome 7q31.2, is a tumor suppressor and chloride channel regulator whose dysfunction disrupts ion transport, leading to thick mucus in lungs and digestive tracts. However, karyotype analysis reveals that over 2,000 distinct mutations in CFTR have been identified, with the most common being ΔF508—a deletion causing misfolded protein and premature degradation.
Yet, these mutations vary across populations, underscoring the importance of comprehensive karyotyping in diagnosis and personalized treatment.
The Genetic Architecture of Cystic Fibrosis Karyotype
The CysticFibrosisKaryotype reveals a mosaic of genetic variation central to clinical outcomes. While ΔF508 homozygosity remains the prototypical pathogenic allele, gene sequencing has uncovered genotype-phenotype correlations that redefine traditional assumptions. For instance, compound heterozygosity—carrying two different CFTR mutations—often produces more severe disease than single homozygous variants, especially when both mutations disrupt critical domains of the CFTR protein.This insight reshapes genetic counseling, as karyotype results now guide not only diagnosis but prognosis and therapeutic planning.
- Adenine-Nucleotide Polymerase (ANP), involved in DNA replication, has been linked to replication fidelity near CFTR, though its direct role in cystic fibrosis remains investigational. - Structural variations such as inversions, duplications, and copy number variants (CNVs) further expand the karyotypic spectrum. - While point mutations dominate, large deletions or insertions account for minor but significant clinical variations.Such discoveries underscore that cystic fibrosis karyotypes are not static; they are dynamic maps reflecting biological complexity.
Diagnostic Precision and Karyotype Testing in Cystic Fibrosis
Accurate diagnosis of cystic fibrosis hinges on integrating clinical metrics—such as sweat chloride levels—with molecular karyotyping. Traditional sweat tests remain vital, but genetic karyotyping offers superior specificity.By analyzing the CFTR gene locus through next-generation sequencing (NGS) panels, clinicians can pinpoint exact mutations, identify at-risk carriers, and anticipate disease progression. For example, the presence of combination mutations like G551D with reduced-function variants predicts responsiveness to modulator therapies like ivacaftor and elexacaftor.
Karyotyping also distinguishes cystic fibrosis from similar conditions with overlapping symptoms, such as primary ciliary dyskinesia or congenital chloride transport defects.
A negative karyotype for known CFTR mutations, paired with elevated immunoreactive trypsinogen (IRT), prompts expanded genetic evaluation, preventing misdiagnosis and inappropriate treatment.
Quotes from clinical experts emphasize the transformative impact: “Precision karyotyping has shifted cystic fibrosis from a one-size-fits-all condition to a spectrum of tailored clinical pathways,” says Dr. Elena Márquez, a geneticist at the Cystic Fibrosis Foundation’s certified lab. This reclassification enables earlier interventions, improved quality of life, and optimized use of high-cost, targeted lifesaving drugs.Carrier Screening and Population Genetic Insights
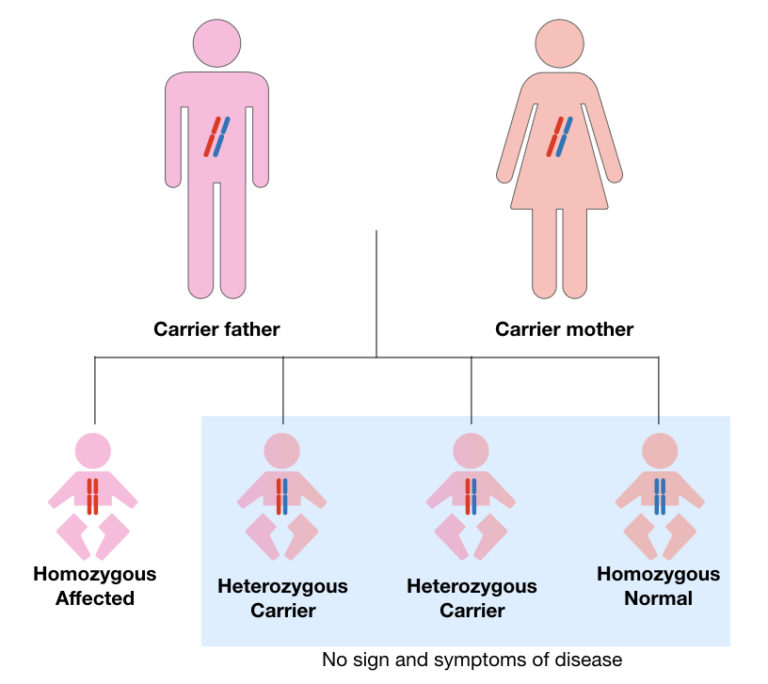
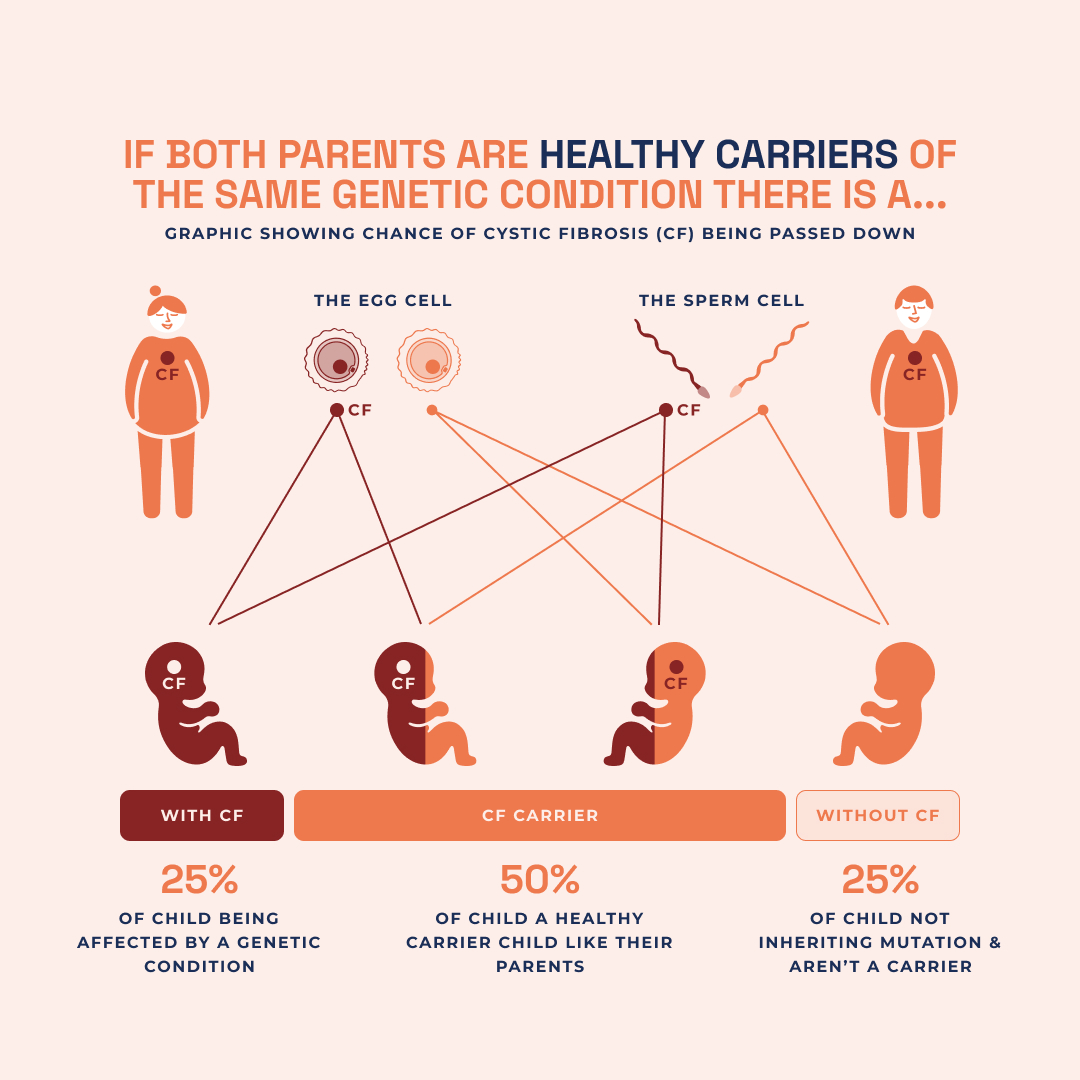
Carrier frequency varies dramatically across ethnic groups, with heterozygote rates reaching 1 in 25 among Caucasians—compared to negligible levels in African and Asian populations. Newborn screening programs worldwide increasingly incorporate CFTR karyotyping to identify asymptomatic carriers, facilitating reproductive planning and preimplantation genetic diagnosis. Population-based karyotyping data also illuminate founder mutations, such as the common ΔF508 homozygosity in Northern Europeans, enhancing targeted screening strategies. Ethnic variation further influences carrier testing algorithms.In regions with high genetic diversity—including African diaspora communities—expanded mutation panels are essential to capture rare but pathogenic variants. Here, karyotype analysis serves as both a preventive tool and a bridge to equitable healthcare access.
Emerging Frontiers: From Karyotype to Personalized Therapy
As gene editing technologies like CRISPR-Cas9 mature, the CysticFibrosisKaryotype transitions from diagnostic benchmark to therapeutic roadmap.Understanding the precise CFTR mutation profile enables corrective gene editing—correcting ΔF508 misfolding, restoring folding machinery, or replacing defective alleles. Clinical trials are now testing virus-delivered gene therapies designed to target specific karyotypic mutations, promising durable correction where standard treatments offer only symptomatic relief.
“Each CCYTK variant represents not just a diagnosis, but a therapeutic target,” articulates Dr. Rajesh Patel, lead investigator in a landmark gene therapy trial.“By decoding the karyotype at this granular level, we move closer to a future where cystic fibrosis becomes a manageable condition, not a life-limiting one.” Beyond gene editing, karyotype-driven pharmacogenomics continues to refine treatment choice. Cocktail therapies—combination modulators tailored to mutant CFTR proteins—now rely on accurate karyotyping to match patients with optimal drugs, reducing adverse effects and improving outcomes.
The Future of Cystic Fibrosis: Karyotype as a Gateway to Progress
The CysticFibrosisKaryotype is no longer a static genetic report but a dynamic catalyst for innovation.From pinpointing rare mutations and enabling precision care to guiding carrier screening and accelerating gene therapy, karyotype analysis integrates biological detail with clinical impact. As sequencing costs fall and technology advances, comprehensive karyotyping is becoming standard, empowering every stage of cystic fibrosis management. Through precise genetic insight, researchers and clinicians are rewriting the narrative of this once-feared disorder—one CCYTK at a time.
This evolving understanding not only transforms patient outcomes but offers a blueprint for tackling complex inherited diseases across medicine.


Related Post

How to Download Roblox on School Computers: Navigating Security, Access, and Compliance

Unlocking the Posterior Cavity: The Hidden World Beneath the Brain’s Base

Washington DC Unemployment: Navigate Login & Benefits with Confidence – Your Step-by-Step Guide

Sabrina Carpenter Body Measurements: A Detailed Breakdown of Her Physique and Public Profile

