Does a Liquid Have a Definite Volume? The Science Behind Flowing Clarity
Does a Liquid Have a Definite Volume? The Science Behind Flowing Clarity
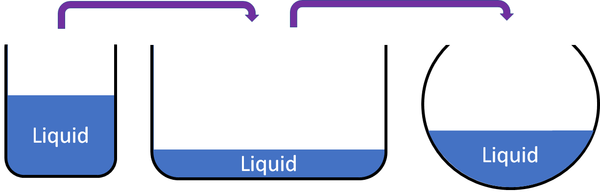
At first glance, liquids appear to embrace the fluidity of their form—unfettered, ever-changing, impossible to pin down. Yet, in the precise world of physics, the question lingers: Does a liquid truly possess a definite volume? While liquids flow gracefully under pressure, their physical behavior reveals a consistent foundational trait—volume remains constant under stable conditions.
This distinction separates mere liquids from behaving fluids, revealing how molecular structure governs measurable reality.
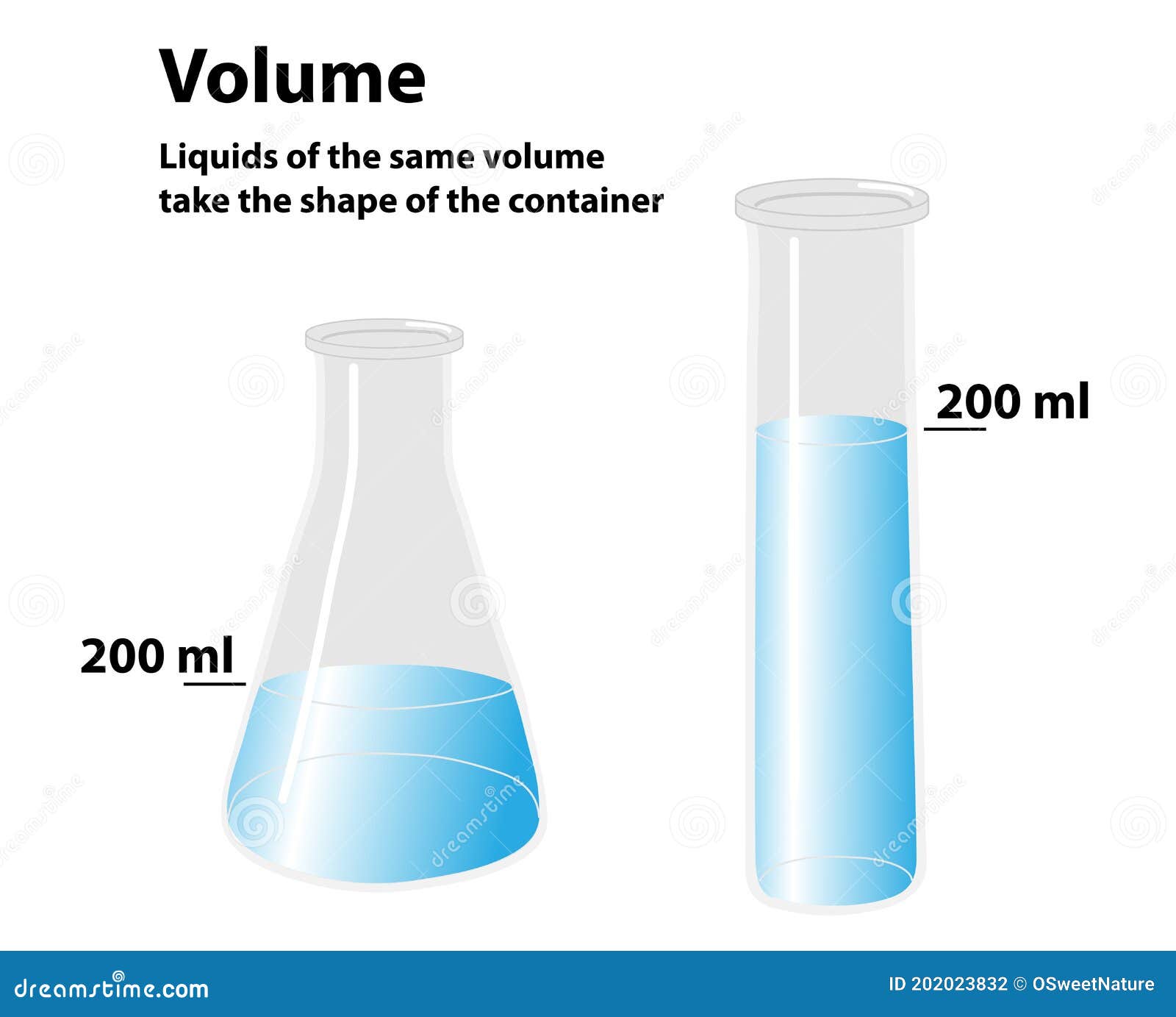
Water, the archetypal liquid, exemplifies this principle. Whether poured into a cup, pooled across a rocky surface, or held in a beaker, its volume does not alter sharply with shape or container. A full liter measured in a lab remains a liter, regardless of the vessel’s geometry.
“Liquids resist compression and maintain volume through cohesive intermolecular forces,” explains Dr. Elena Marquez, a physical chemist at MIT. “Their molecules are close-packed but constantly in motion, allowing them to adapt to containers while preserving total mass and, therefore, volume.”
Defining Volume in Fluids: A Matter of Constancy and Measurement Volume in liquids is fundamentally defined by the sum of the volumes of their constituent molecules.
Unlike gases, where individual particles roam freely and compressibility distorts volume under pressure, liquid molecules remain in near-proximity due to stronger intermolecular bonds. This molecular order enables predictable, repeatable volume measurements—critical across scientific and industrial applications. Nations rely on standardized cubic meters for trade, a testament to liquids' reliable volume.
As the International Bureau of Weights and Measures notes, “In metrology, a liter is preserved as a fixed volume under controlled temperature, underscoring liquid volume’s objective reality.”
But liquid volume’s constancy under fixed conditions contrasts with variable behavior in changing environments. Temperature and pressure significantly influence molecular motion, which in turn affects volume. Warm water expands slightly as kinetic energy increases—expanding container boundaries may reveal a volume shift, though the liquid’s intrinsic volume measure remains unchanged.
“A detector may observe expansion, but the substance’s fundamental volume isn’t lost,” clarifies Dr. Marquez. “Volume defines quantity per unit mass and under specific physical conditions, not absolute immobility.”
Closely tied to volume is density—a ratio defining mass per unit volume—which adds layers to liquid behavior.
A liter of mercury, denser than water, occupies the same volume yet carries greater mass, explaining its weight and practical use in barometers. These relationships, documented since Archimedes’ principle, reinforce that volume is not an isolated property but part of a dynamic system of physical constants. Liquid volume thus serves as a measurable benchmark in chemistry, engineering, and everyday science.
In industrial and laboratory settings, this stability enables precision.
Imagine fabricating pharmaceuticals: a consistent 500 mL of solution ensures reliable dosing, no matter the container shape. Alternatively, in climate science, tracking oceanic liquid volume changes aids sea-level rise predictions—volume remains uniform at set temperatures, allowing scientists to isolate thermal expansion effects. “Precision relies on treating liquids as uniform volume units across diverse conditions,” states an engineer at NASA’s flow dynamics lab.
“Whether measuring coolant in spacecraft systems or rainwater runoff, consistent volume values anchor accurate data.”
Paradoxically, liquids’ reputed “flowability” masks this hidden order. Their surface tension allows them to conform to shapes effortlessly, but beneath this fluid grace lies structural rigidity. “They flow because molecules can slide, yet their total volume—accountable through calibration and measurement—remains fixed,” adds Dr.
Marquez. “This duality separates fluid motion from volume instability—a key concept in understanding real-world fluid behavior.”
Many mistakenly assume liquids shift volume arbitrarily—a belief fueled by rapid spreading or mixing. Yet true volume is conserved in static, controlled environments.
When oil spreads on water, the total volume doesn’t expand—instead, interfaces redistribute mass and area, preserving the system’s inherent quantity. As physicist Dr. James Lin asserts, “Liquids demonstrate no ‘loose’ volume; they deliver exact, reproducible measures grounded in molecular cohesion and temperature-stabilized conditions.”
In essence, a liquid does possess a definite volume—consistent, measurable, and indispensable to scientific and practical endeavors.
This constancy shapes fields from medicine to meteorology, proving that behind flowing motion lies a stable, defined property. Understanding this principle deepens both scientific inquiry and everyday awareness—for anywhere liquids flow, a definitive volume still defines their essence.
From water in a dropper to coolant in engines, the liquid’s reliable volume underpins precision across technology and nature. It is this steadfast measurable quality—unaffected by shape, yet unyielding in quantity—that makes volume a cornerstone of physical science.
No flowing essence, no


Related Post

No News Is Not Always Good News: How Absence of Announcements Shapes Perception

One Semi-Foreign Foot: How 75 Cm Shapes Global Measurement Standards
Understanding the User Agent Header: Decoding Browser Signals with Precision

Unlocking the Molecular Blueprint: The Lewis Dot Structure of CH₂O and What It Reveals

