Etc Reactants: The Silent Engine Driving Chemical Transformations
Etc Reactants: The Silent Engine Driving Chemical Transformations
In the heartbeat of every chemical reaction lies an unseen but essential cast of molecules—reactants—whose precise roles determine whether a transformation occurs, how swiftly it proceeds, and what products emerge. Unlike the dramatic imagery of transition states or the fleeting existence of intermediates, reactants are the foundational actors, the starting materials that set motion in motion. Commonly overlooked in favor of catalysts or energy inputs, reactants are the true fuel of chemistry—without them, even the most powerful catalyst or optimal temperature cannot spark change.
Understanding et now reactants is key to mastering both industrial processes and biological systems.
Etc reactants, in scientific discourse, represent the complete set of substances required at the onset of a reaction. These components—whether single molecules, ions, or complex compounds—collide, interact, and undergo transformation to yield products.
The choice and concentration of reactants directly influence reaction yield, selectivity, and efficiency, making them indispensable in fields ranging from pharmaceuticals to materials science. “The selected reactants dictate not just the direction of a reaction, but its very feasibility,” notes Dr. Elena Márquez, a chemical engineer specializing in reaction optimization.
“Even a minor change in reactant purity can shift a high-output process into inefficiency.”
The Core Roles of Etc Reactants in Chemical Processes
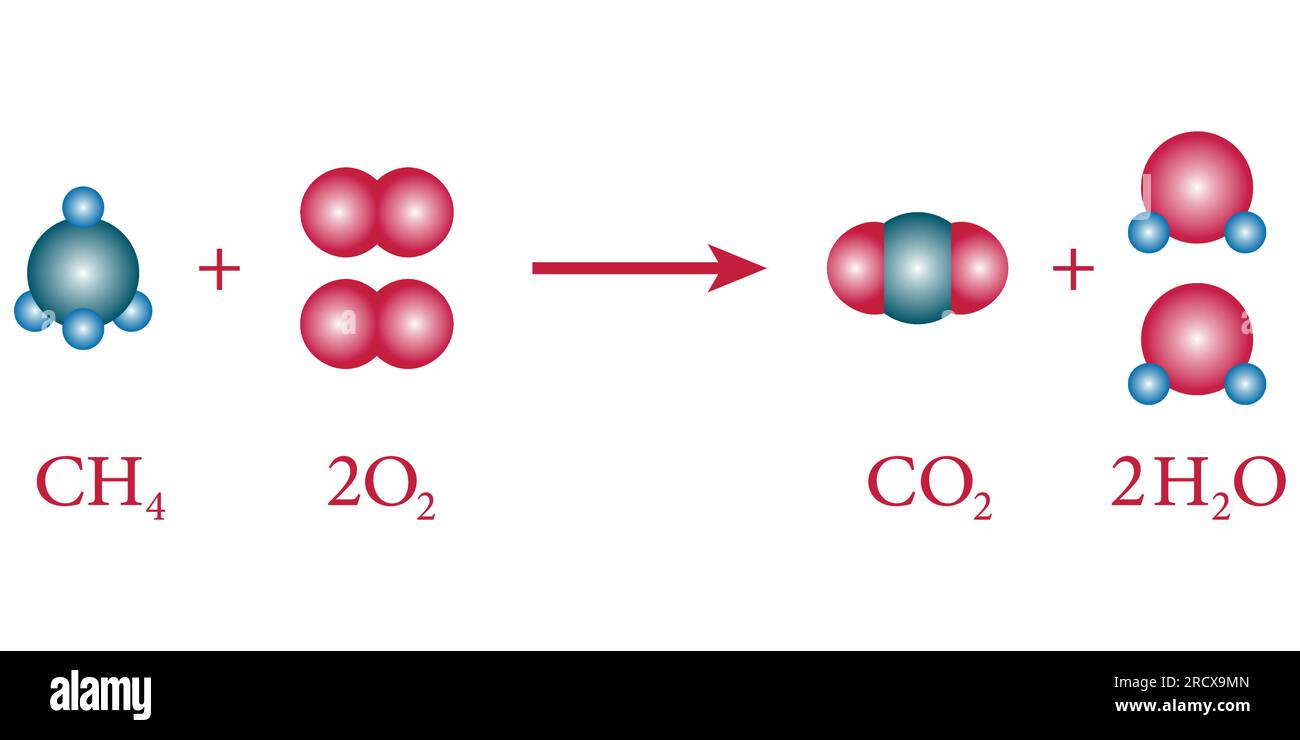
Reactants serve multiple critical functions in chemical dynamics. They initiate reactions by bringing reactive sites into proximity, enabling bond breaking and formation. Their stoichiometric balance determines whether a reaction proceeds toward completion or stalls, governed by the law of conservation of mass.In exothermic reactions, reactants supply the energy released, while in endothermic ones, external energy overcomes their activation barriers. - **Energy Providers**: Reactants often carry latent energy—chemical, electromagnetic, or thermal—that drives transformations. For instance, in combustion, hydrocarbons and oxygen react, releasing energy stored in covalent bonds.
- **Mass Contributors**: The total mass of reactants equates to the mass of products via atomic rearrangement, a principle central to stoichiometry. - **Regulators of Velocity**: High reactant concentration increases collision frequency, accelerating reaction rates in unitary processes.
How Reactant Selection Impacts Industrial and Biological Efficiency
In large-scale manufacturing, choosing the right et c reactants is not just a chemical decision—it’s an economic imperative.“In petrochemical refining, carefully selected hydrocarbon feedstocks maximize conversion efficiency and minimize waste,” explains Dr. Rajiv Patel, a process chemist. “Even trace impurities in reactants can ruin catalysts, halting production lines.” In biochemistry, enzymes act as biological catalysts but rely on precise reactant availability.
For metabolic pathways like glycolysis, glucose, fructose, and ATP function as key reactants; insufficient levels stall energy production at the cellular level. This sensitivity underscores a fundamental truth: reactants define the feasibility and sustainability of chemical transformations. In energy systems, for example, renewable hydrogen as a reactant in fuel cells demands purity and consistent supply to avoid degradation.
In laboratory synthesis, high-purity carbon tetrachloride may determine whether a cross-coupling reaction yields the target compound or defeats with byproducts.
Beyond composition, reactant form and physical state shape reaction behavior. In gas-phase reactions, molecular dissociation enables faster adsorption on catalysts; in aqueous solutions, ionic dissociation enhances solubility and reactivity.
These variables demand meticulous control—industry best practices mandate rigorous quantum-chemical modeling to predict reactant interactions and optimize input quality.
Balancing Act: Stoichiometry, Reactants, and Sustainable Chemistry
The stoichiometric relationship between reactants is not merely a mathematical equation—it’s the blueprint for efficient, green chemistry. The ideal mole ratio maximizes yield while minimizing excess, reducing raw material waste and environmental footprint. “Modern chemistry pivots on precision reactant utilization,” asserts Dr.Márquez. “This precision cuts costs, preserves resources, and strengthens sustainability.” Real-world examples illustrate this principle: in ammonia synthesis (Haber–Bosch process), nitrogen and hydrogen react in a precise 1:3 ratio—not only to maximize yield but to reduce energy demand. Similarly, in pharmaceutical synthesis, using optimal reactant proportions enhances drug purity and lowers purification expenses.
As the field evolves, computational tools now predict optimal reactant mixtures, accelerating discovery while conserving materials. \langleh2>The Essential Synergy: Reactants, Processes, and Innovation Reactants are far more than inert starting materials; they are the linchpins of chemical progress. From industrial scale-ups to cellular machinery, their selection, purity, and stoichiometry govern efficiency, sustainability, and innovation.
As research advances, understanding et c reactants deepens our grasp of transformations that define modern science and industry. Every reaction’s success begins with the careful choice of what reactants are brought together—and how they are harnessed. The future of chemistry lies in mastering these foundational components, turning raw materials into purposeful, scalable, and eco-conscious outcomes.
In the silent beginning of every transformation, reactants speak the language of possibility—one carefully chosen element at a time



Related Post

Discover The Charm Of Maplestar: Your Ultimate Guide to a Uniquely Enchanting Community

Jared Fogles Release Date Revealed: The Full Timeline Confirmed — What Fans Need to Know Now

Jason Kelce’s Retirement Masterpiece: A Fond Honor Beyond the Gridiron

Barnsley vs Rotherham: The South Yorkshire Derby Rivalry Heats Up Ahead of Showdown

