From Desert Survivors to Salt Pans: Real-Life Mastery of Osmosis in Everyday Life
From Desert Survivors to Salt Pans: Real-Life Mastery of Osmosis in Everyday Life
Nature’s most fundamental process—osmosis—governs water movement across semipermeable membranes, shaping everything from cellular hydration to environmental ecosystems. While often discussed in biology labs, osmosis plays a direct, tangible role in daily human experiences, from drinking water in arid climates to preserving food. By observing real-world applications, we gain insight into how osmosis sustains life, protects health, and powers industrial innovation.
This article explores key examples where osmosis shapes outcomes—both invisible and essential.
The Human Eye: Osmosis in Vision Maintenance
Inside the human eye lies a delicate balance maintained by osmosis, critical for clear vision and long-term eye health. The aqueous humor, a clear fluid filling the space between the cornea and lens, relies on osmotic pressure to maintain proper hydration of corneal tissues.Without this balance, corneas can swell, impairing sight. Osmosis here ensures nutrient delivery and waste removal at the cellular level. Specifically, the vitreous humor—comprised mostly of water and gel-like polymers—maintains structural integrity through osmotic gradients.
The concentration of dissolved molecules, including ions and sugars, creates an osmotic force drawing water into the vitreous from surrounding tissues. Disruptions in this balance, such as in glaucoma or diabetic eye disease, compromise tissue function. “The eye’s reliance on osmotic stability underscores osmosis as a silent guardian of vision,” explains ophthalmologist Dr.
Elena Ruiz. “Even minor shifts in fluid dynamics can threaten sight—making every osmotic adjustment a vital physiological event.” Patients with cataracts or those undergoing intraocular pressure treatment frequently depend on osmotic principles. For instance, pressures from overproduced aqueous humor disrupt osmotic equilibrium, contributing to clouded vision.
Surgeons counter such imbalances using medications that adjust fluid dynamics, stabilizing osmotic forces to protect lens clarity.
Renal Osmoregulation: The Kidney’s Osmotic Powerhouse
The kidneys stand as biological masterpieces of membrane-based regulation, where osmosis enables precise control of water and electrolyte balance. As blood filters through nephrons—the functional units of the kidney—osmosis drives the selective reabsorption of water.This process ensures that Dawson’s law governs every drop: the renal tubules generate hyperosmotic medullary fluid, concentrating urine while conserving vital water. “Without osmosis, the kidneys couldn’t transform excess fluid into concentrated urine, risking severe dehydration or fluid overload,” notes nephrologist Dr. Raj Patel.
In healthy adults, osmotic gradients created by transported sodium and urea allow 99% of filtered water to be recycled. When dehydration strikes, antidiuretic hormone (ADH) increases water permeability in collecting ducts, intensifying osmosis and reducing urine output. In contrast, overhydration triggers reduced ADH, allowing more water to move into cells via osmosis, preventing dilution of critical blood electrolytes.
Real-life implications are profound. Patients with kidney disease often suffer from osmotic imbalances—failing to concentrate urine leads to dangerous fluid swings. Treatments such as osmotic diuretics (e.g., mannitol) exploit osmotic pressure to draw excess fluid from tissues, offering rapid relief in critical care.
Salt Pans: Nature’s Osmotic Factories
In arid landscapes, evaporative ponds known as salt pans offer a dramatic demonstration of osmosis in action. As water evaporates under intense sun, remaining brines grow increasingly concentrated, triggering selective osmotic crystallization. Different salts precipitate at distinct saturation levels—first sodium chloride, then magnesium and calcium salts—driven not by temperature but by osmotic forces governing solute mobility.“Osmosis dictates which ions deposit first, sculpting the layered mineral hospitals seen in deserts like The Salar de Uyuni in Bolivia,” describes geochemist Dr. Maria Chen. In these basins, hypersaline conditions push microbial communities to adapt or perish, creating ecosystems shaped by osmotic limits.
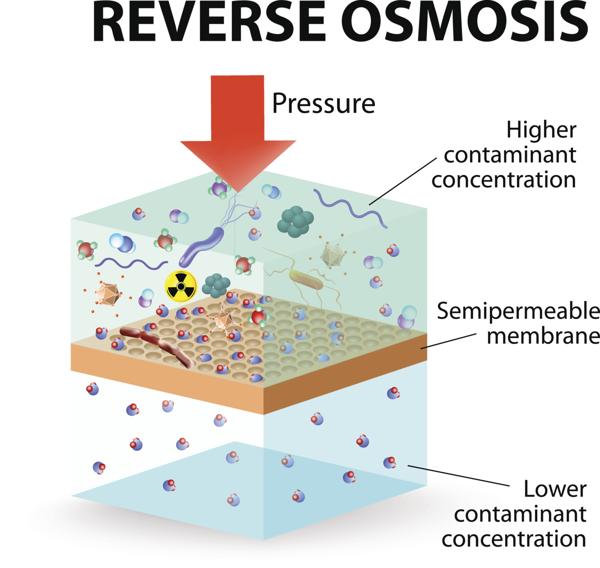
Microbes producing compatible solutes—such as betaine and glycerol—survive by balancing internal osmotic pressure against the external brine, illustrating evolutionary osmosis. Beyond ecology, salt pans supply raw materials for industry. The concentration gradients enforced by relentless evaporation mirror industrial membrane technologies like reverse osmosis (RO), now pivotal in water purification and desalination globally.
Cellular Hydration: Osmosis as Life’s Liquid Foundation
At the micro level, osmosis governs cellular function in a battle for equilibrium. Cells face dual challenges: maintaining turgor pressure to preserve shape and volume, and preventing lysis or crenation in fluctuating environments. Plant cells exemplify this: in hypotonic solutions, water floods in through flexible membranes, swelling to form turgid, rigid structures essential for standing upright.In hypertonic conditions, water exits, causing crenation—cells shrink and lose function. Animal cells face similar stakes. Red blood cells in saline extremes illustrate osmosis’s power: in 0.9% sodium chloride (isotonic), they remain stable; in 0.45% (hypotonic), they bloat; in 3% (hypertonic), they shrink.
Medical protocols use isotonic saline infusions to safely restore blood volume without damaging cells—a routine application where precise osmotic control saves lives. Even neurons depend on osmotic vigilance. The blood-brain barrier tightly regulates solute gradients, ensuring the brain’s delicate fluid environment remains stable against systemic swings.
“Every breath, every clutch of water, every nutrient absorbed hinges on osmosis fine-tuned to cellular precision,” states Dr. Li Wei, a cellular physiologist. Disruptions—such as in brain edema—reveal how critical osmotic balance is to neural health.
Food Preservation: Osmosis as a Natural Preservative
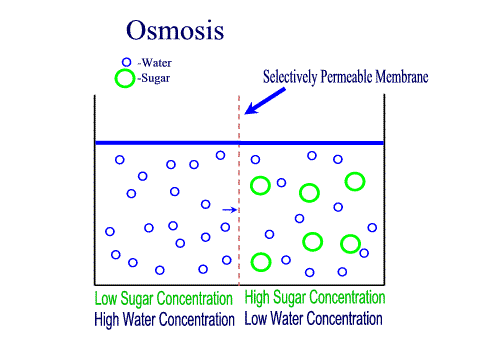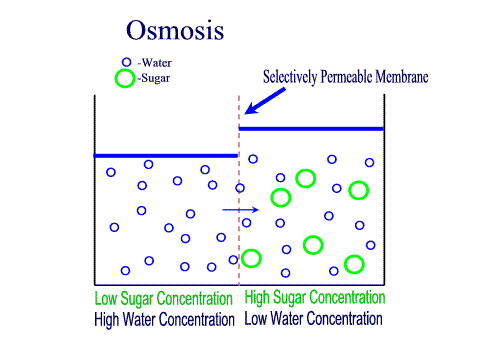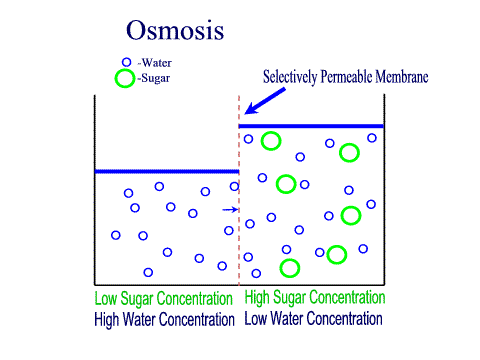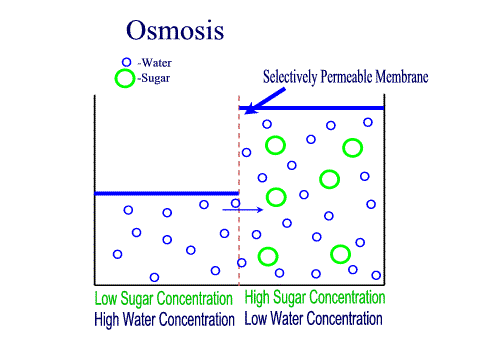
Tradition and science converge in food preservation techniques rooted in osmosis. Salting, sugaring, and drying exploit osmotic gradients to draw moisture from perishables, creating inhospitable environments for bacteria and fungi. Jams and jellies rely on high sugar concentrations to lower water activity; microbes starve as osmotic pressure pulls hydration from their cells.Pickling extends this principle: vegetables soaked in brine


Related Post

Big Breakfast, Big Numbers: Decoding McDonald’s Iconic Morning Meal Calorie Count and Nutrition Facts

Dawn Hasbrouck Age: Unraveling the Legacy of a Trailblazing Voice in Public Life
Summer And Morty: The Unmatched Dynamic That Defined an Animated Frontier

Dyrroth vs Alucard: The Clash of Titans in Mobile Legends’ Lethal Mobile Showdown

