Halogens: The Reactive Champions of the Periodic Table
Halogens: The Reactive Champions of the Periodic Table
From fiery volcanic eruptions to the precise chemistry of life-sustaining molecules, halogens stand out as the electrifying idealists of the periodic table—highly reactive, electron-hungry, and essential to countless natural and industrial processes. As the seventh group (Group 17) elements, fluorine, chlorine, bromine, iodine, and astatine exhibit a shared mastery of valence electron dynamics, making them indispensable across science, medicine, and environmental systems. Their positions on the periodic table—fluctuating electronegativity, decreasing atomic radius across the group—dictate their behavior, from aggressive nucleophilicity to subtle biological integration.
This article explores the halogens’ defining characteristics, their remarkable periodic trends, diverse applications, and their enduring significance in both natural and human-engineered contexts.
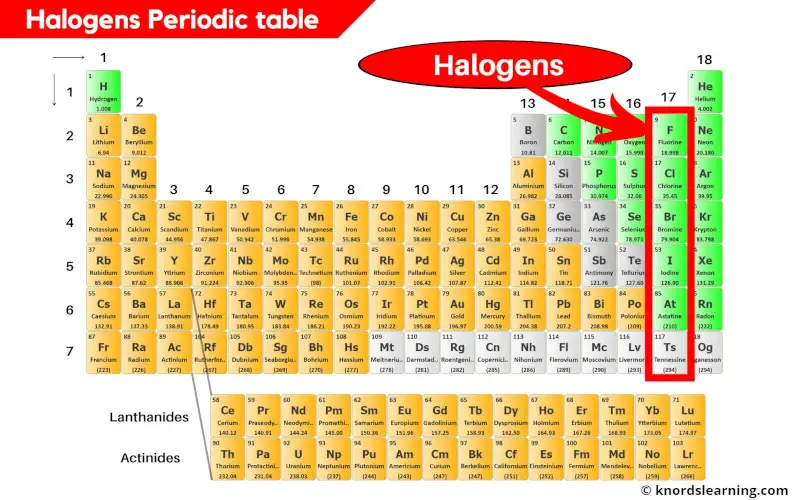
Core Properties and Periodic Trends of Halogens
Hydrogen defies the group as a non-halogen, yet its proximity sets the stage for halogen dominance in chemical behavior. Group 17 elements share critical features: each possesses seven valence electrons, driving an unrelenting quest to gain a single electron for complete stability, resulting in one-negative ion charge (X⁻) in compounds.As one moves down the group—fluorine (F) to astatine (At)—atomic size increases significantly due to additional electron shells, and atomic mass rises substantially. This growth correlates directly with weakening electronegativity: fluorine, the most electronegative element (χ = 3.98), males chlorine (3.16), bromine (2.96), iodine (2.66), and astatine (2.2)—a trend that shapes reactivity. Electronegativity and Bonding Behavior: Fluorine’s dominance in electron attraction enables powerful hydrogen bonding, explaining water’s unique properties and why HF is the strongest acid among typical hydrogen halides.
Across the group, bond dissociation energies decline—stronger F–F bonds (158 kJ/mol) give way to weaker At–At bonds (106 kJ/mol)—a reflection of increasing atomic volume and reduced bond density. The halogens also display varied oxidation states: while F⁻ is exclusive and stable, chlorine permanges from –1 to +7; bromine ranges –1 to +5; iodine spans –1 to +7; and astatine exhibits the broadest but least studied range up to +5 or +7. The fluorous factor—fluorine’s unmatched electronegativity—makes it irreplaceable in extreme environments, influencing bond stability, acidity, and reactivity in flame retardants and surgical implants.
Reactivity and Role in Chemical Transformations
Halogens dominate chemical reactivity in unique, predictable ways, acting as both oxidizing agents and nucleophiles. Their hallmark is a fierce tendency to acquire an electron, manifesting in vigorous reactions with metals (e.g., Mg + 2Cl₂ → MgCl₂) and hydrogen (e.g., \( \text{H}_2 + \text{I}_2 \rightarrow 2HI \)). Among the group, fluorine leads in oxidation power, fluorinating nearly all elements, including noble gases like xenon.Chlorine excels in disinfection and organic chlorination, while bromine’s lower reactivity suits safer industrial applications like flame retardants and UV absorbers. Halogen chemistry underpins life-sustaining processes. Oxygen and halogens combine to form life-essential hydrogen halides in metabolic pathways, while iodine is vital for thyroid hormone synthesis—each atom’s role precisely tuned by position on the periodic table.
Their redox behavior highlights halogens as central translators between oxidation states, bridging reactive substances in clean energy (e.g., redox flow batteries), environmental remediation, and polymerization catalysts. In essence, halogens are not merely reactive—they are chemical architects.
Applications Across Industries and Daily Life
The halogens’ utility spans medicine, technology, and environmental protection, driven by their distinct physicochemical traits.• Medicine and Healthcare: Iodine is foundational in radiocontrast agents for medical imaging and antiseptics like povidone-iodine. Fluorinated compounds, especially those incorporating ¹⁹⁹F or ¹⁸F, power positron emission tomography (PET) scans, enabling precise tumor detection across oncology. Fluoride ions strengthen tooth enamel, preventing decay in community water supplies since the 20th century.
• Industrial and Material Science: Chlorine is the backbone of PVC piping, disinfectants, and chemical feedstocks. Brominated flame retardants prevent fires in electronics, furniture, and textiles—though environmental persistence raises recent concerns. Iodine compounds serve in pharmaceuticals (amoxicillin derivatives) and sterilization.
• Everyday Products: Hydrofluorocarbons (HFCs), once chlorine-chlorine hybrids, revolutionized refrigeration and air conditioning, though their global warming potential now drives shifts to hydrofluoroolefins (HFOs). Sea salt aérosols and aerosol sprays utilize chloride nanoparticles for controlled moisture release, demonstrating halogens in consumer wellness. Each molecule reflects halogens’ ability to balance reactivity with utility—used safely when applied with understanding.
Historical Discovery and Environmental Impact
The halogens emerged from 18th- and 19th-century alchemy, with chlorine isolated by Carl Wilhelm Scheele in 1774, fluorspar (CaF₂) identified earlier by Johann Wolfgang Döbereiner, and iodine revealed by Bernard Courtois during gunpowder production (~1811). Astatine, radioactive and rare, was synthesized in the 1940s but remains poorly studied due to limited availability. Their industrial rise brought unintended consequences.Mobile halogen compounds like CFCs eroded the ozone layer, prompting the Montreal Protocol (1987), a landmark global treaty phasing out hazardous members. Meanwhile, radioactive astatine and brominated flame retardants now challenge waste management and ecological safety. These stories underscore halogens’ dual role: powerful tools and environmental stewards in delicate balance.
The Future of Halogens: Innovation and Sustainability
As science advances, halogens remain at the forefront of innovation. Perfluoroalkyl and polyfluoroalkyl substances (PFAS), long used in non-stick coatings and firefighting foams, face growing scrutiny for environmental persistence. Researchers now focus on safer fluorine alternatives and greener synthesis methods.In materials science, halogen-based perovskites promise high-efficiency solar cells, leveraging bromine and chlorine’s electronic tuning. Nanotechnology exploits iodine’s biological sensitivity in targeted drug delivery and biosensors. In energy storage, redox-active halogens enable next-gen batteries with high energy density.
The halogens’ journey—from elemental curiosity to industrial backbone, medical marvel, and environmental challenge—reflects humanity’s evolving relationship with chemistry. Their periodicity

Related Post

Decoding the Hybrid Hit: Osclagusc’s Sonic Alchemy Meets Camila Cabello in Havana’s Soundscape

Inside the Digital Heartbeat: How Top Computing Stores Fuel Tech Innovators and Everyday Users Alike
.webp)
Start Your Day Right: What To Read Instead Of News

Wyoming in August: A Season of Golden Highs and West Winds

