How Covalent Bonding with Fluorine F2 Drives Revolutionary Chemical Advances
How Covalent Bonding with Fluorine F2 Drives Revolutionary Chemical Advances
Fluorine’s unmatched electronegativity and small atomic radius make it a linchpin in modern chemistry—particularly when forming covalent bonds, especially with hydrogen in F₂ (dichlorine fluoride) and related compounds. While F₂ is famously reactive, its role in stabilizing covalent networks—especially through selective bond formation—has profound implications across pharmaceuticals, materials science, and industrial chemistry. Understanding covalent bonding with F₂ reveals not only fundamental principles but also real-world applications where precision and reactivity converge.
The Chemical Nature of Covalent Bonds in F₂
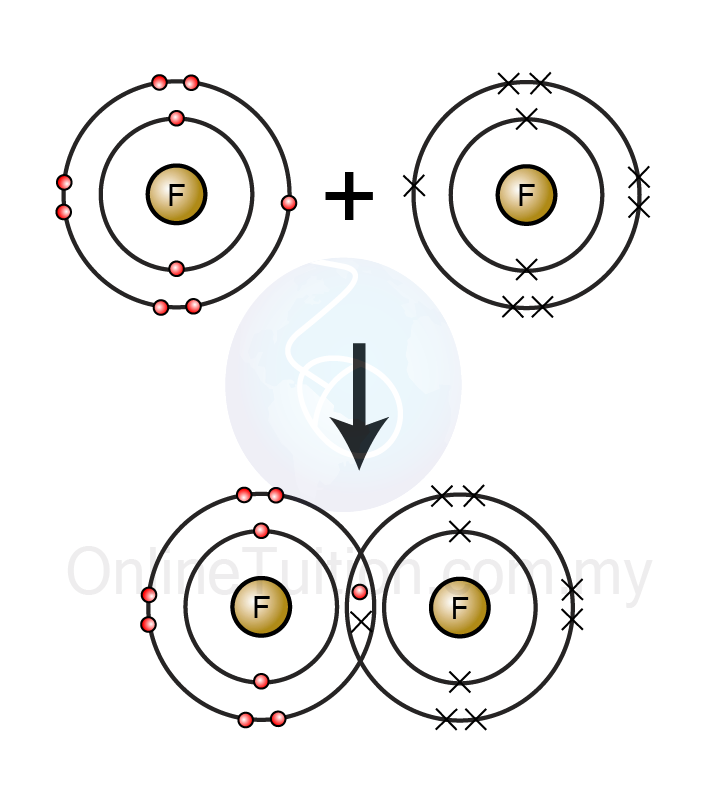
Covalent bonding, defined by the sharing of electron pairs between atoms, lies at the core of F₂’s stability and reactivity. In the diatomic fluorine molecule (F₂), two fluorine atoms share a single pair of electrons, forming a strong, directional single bond. Yet, F₂ itself is a highly electrophilic species—mutually electron-deficient at each atom—making it a powerful reagent rather than a passive bonded unit.This duality—stable bond within F₂, yet extreme reactivity in chemical transformations—underpins its utility. The covalent asymmetry in F₂ means each fluorine atom carries a partial positive charge, while the shared electrons reside closer to the smaller fluorine atom. This electronic distribution influences how F₂ interacts with other molecules: its electrophilic character enables it to initiate electron-transfer reactions and activate substrates, particularly in nucleophilic addition and polar bond cleavage processes.
Understanding F₂’s co-d bonding behavior—even within the molecule itself—provides insight into how such hyper-reactive species can be harnessed safely in controlled environments. Unlike free fluorine gas, which is notoriously destructive, covalent installations like those studied in F₂ enable selective, predictable reactivity vital for synthetic design.
Electronegativity and Bond Strength: The Role of Fluorine’s Fluorine
Fluorine’s exceptional electronegativity—measured at 3.98 on the Pauling scale—directly governs the character of covalent bonds it forms. When bonding with hydrogen or carbon, fluorine preferentially draws electron density, creating polar covalent interactions that enhance bond stability in downstream applications.In F₂, the F–F bond is unusually strong at room temperature (bond energy ~158 kJ/mol), a rarity among diatomic halogens.
This robust F–F bond arises from effective p-orbital overlap and partial electron sharing, stabilized by fluorine’s small size, which minimizes orbital repulsion and maximizes bond covalency. Despite F₂’s overall high reactivity, the intramolecular bond remains relatively inert outside extreme conditions, preserving the molecule’s structural integrity in specialized contexts.
Covalent Bonding Mechanisms in Fluorinated Compounds Beyond F₂
While F₂ itself is a diatomic molecule, the principles of covalent bonding it exemplifies extend to more complex fluorinated systems—critical in drug development, polymers, and plant biochemistry.In ethyl fluoride (CH₃CH₂F), for example, the C–F bond demonstrates strong covalent character, contributing to the compound’s stability and lipophilic properties. Similarly, in nucleosides, fluorine atoms often form covalent linkages that fine-tune biological activity without compromising molecular integrity. F₂ serves as a model for studying such bond formation.
Researchers observe that controlled covalent interactions with fluorine often require precise activation—whether through catalytic surfaces, directing groups, or solvent effects—to prevent uncontrolled chain reactions. The mechanism typically involves initial electrophilic attack at the fluorine atom, followed by stepwise electron redistribution, culminating in stable covalent networks tailored for functionality.
Industrial processes exploit this knowledge: fluorinated polymers leverage covalent C–F bonds for thermal and chemical resistance, while pharmaceuticals use selective fluorination to boost metabolic stability and binding affinity.
Applications Driving Innovation Across Sectors
The controlled covalent bonding of F₂-inspired fluorine derivatives powers breakthroughs in multiple domains: - **Pharmaceuticals**: Fluorine incorporation enhances drug longevity and target selectivity.Covalent fluorinated analogs, derived from understanding F₂’s reactivity profile, reduce degradation in biological systems, improving dosage efficacy and reducing side effects. - **Materials Science**: Fluorinated coatings and covalent networks yield coatings with unmatched durability, repellency, and electrical insulation—essential for aerospace and microelectronics. - **Agrochemicals**: Fluorinated pesticides and herbicides exploit covalent stability to extend environmental persistence and target precision, boosting crop protection while minimizing ecological runoff.
“Fluorine’s role isn’t just



Related Post

PuppetKillerUnblocked

Bloodhouth Lil Jeff Death Video: The Dark Unraveling Behind a Tragic Moment That Shook the Rap Community

Shilo and Shedeur Sanders: The Sanders Sons Step Into the Spotlight—A New Generation Rises

Mastering the HR Playbook: How AttHrOneStop Transforms Workplace Administration

