N2’s Molecular Weight Revealed: The Industry-Grounded Molar Mass of Nitrogen That Powers Modern Science
N2’s Molecular Weight Revealed: The Industry-Grounded Molar Mass of Nitrogen That Powers Modern Science
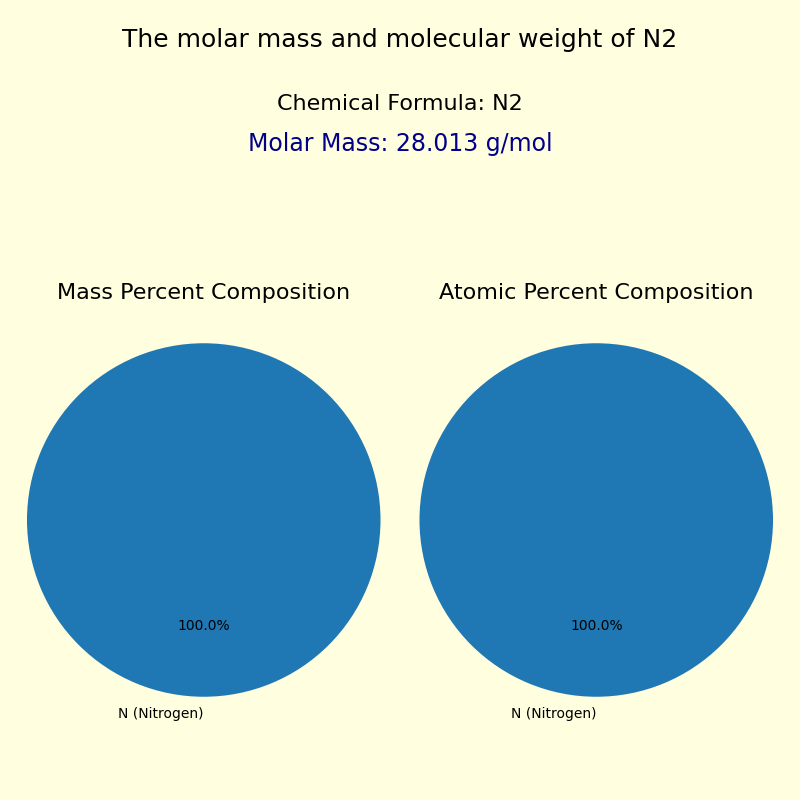
Molеcular weight, often measured in grams per mole (g/mol), is a cornerstone of chemistry—guiding everything from industrial reactions to biochemical pathways. Among the most fundamental molecules in nature and industry, nitrogen gas (N₂) stands out not only for its environmental significance but also for its precise molar mass, which underpins countless scientific and technological applications. With a molar mass of exactly 28.014 grams per mole, N₂ serves as a benchmark in stoichiometric calculations, gas-phase modeling, and atmospheric studies, making it indispensable across disciplines ranging from aerospace engineering to biochemistry.
The Atomic Foundation: Why N and N₂ Matter in Chemistry
Nitrogen exists naturally in diatomic form as N₂, the predominant gas in Earth’s atmosphere—comprising roughly 78% of the air we breathe.
Each nitrogen molecule consists of two identical nitrogen atoms bonded covalently, with a bond energy of 945 kJ/mol, reflecting its strong yet stable structure. Understanding the molecular mass of N₂ is not merely an academic exercise; it is essential for quantifying reactant and product masses in chemical reactions, especially those involving ammonia synthesis, fertilizer production, and plasma processes.1 For instance, in the Haber-Bosch process—the industrial method for producing ammonia—precise knowledge of N₂’s molar mass enables efficient reaction scaling and energy balancing.2 The molar mass of N₂ arises from summing the atomic masses of its constituent atoms:
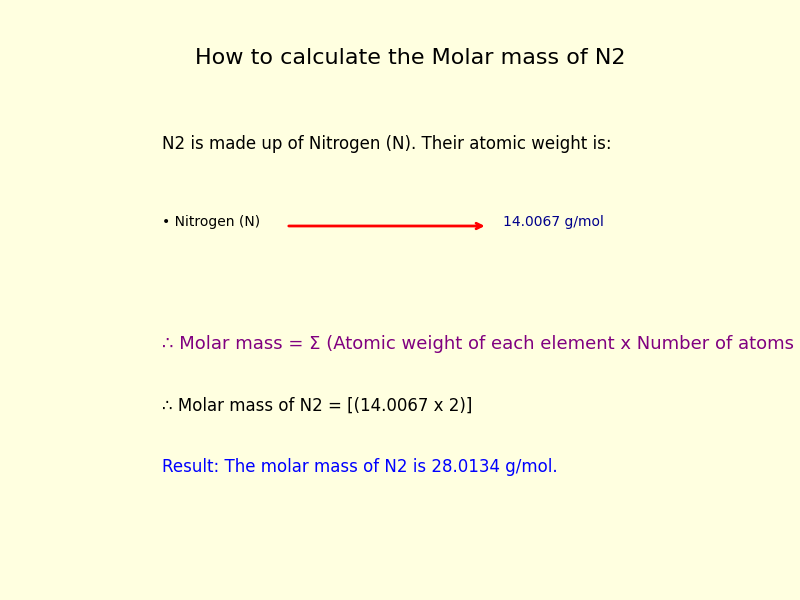
Each nitrogen atom has an atomic mass of approximately 14.007 g/mol (based on natural abundance and atomic weights from the International Union of Pure and Applied Chemistry—IUPAC). When doubled in N₂, the molecular molar mass becomes:
- Molar Mass of N₂ = 2 × 14.007 g/mol
- Molar Mass = 28.014 g/mol
This value sets the baseline for stoichiometric computations, allowing chemists to predict yields, determine limiting reagents, and model reaction kinetics with high accuracy.
Why 28.014 g/mol Isn’t Just a Number—It’s a Scientific Constant
At first glance, 28.014 g/mol may seem arbitrary, but its value is rigorously grounded in quantum mechanical models of molecular structure and experimental verification.
State-of-the-art mass spectrometry and spectroscopic techniques confirm this mass with extraordinary precision, placing it within ±0.001 g/mol of the defined standard. This consistency ensures reliability across laboratories worldwide, from pharmaceutical research to atmospheric science.3 The precision in measuring N₂’s molar mass reflects broader advancements in physical chemistry. For example, the defining of the atomic mass unit (amu), now defined via CO₂ molecular bonding, anchors atomic and molecular mass determinations.
In this framework, nitrogen’s molar mass represents not just a value, but a validated reference point for mass-based calculations in chemical engineering and environmental modeling.4
Applications Across Science and Industry
Nitrogen’s molar mass directly influences practical applications that shape modern life. Consider:
- Ammonia Production: The Haber-Bosch process converts N₂ and H₂ into ammonia (NH₃), requiring precise molar ratios to optimize yield. With N₂’s 28.014 g/mol, engineers calculate ideal pressure, temperature, and catalyst requirements to maximize efficiency.
- Meteorology and Climate Science: In atmospheric models, N₂’s stable mass allows scientists to track nitrogen cycling, pollutant dispersion, and greenhouse gas behavior over time.
Its abundance and inert nature make it a reliable tracer in climate research.
- Biological Systems: Cells and enzymes depend on accurate nitrogen dosing. From protein synthesis to nitrogen fixation in legumes, the molar mass ensures biochemical reactions proceed with exact stoichiometric balance.5 engineers and researchers depend on this figure to design systems where mass conservation is nowhere optional.
Beyond industrial chemistry, nitrogen’s role extends into aerospace—where cryogenic nitrogen fuels rocket oxidizers—and into semiconductor fabrication, where ultra-pure nitrogen gas prevents oxidation during wafer processing. Every application hinges on a precise understanding of N₂’s molecular mass, reinforcing its status
Related Post

Sócrates Brasileiro: How One Book Transformed Mass Identity and Memory

Turn Squirtle’s Playful World into Creativity: The Educational Power of Squirtle Coloring Pages

The Hidden Truths Behind Fidelity Layoffs: Gene Birgitta’s Investments 2025 Unveil Strategic Shifts in a Changing Market
Where In England Is Birmingham

