The Ideal Gas Constant: The Key to Decoding Pressure, Volume, and Temperature
The Ideal Gas Constant: The Key to Decoding Pressure, Volume, and Temperature
From weather forecasting to industrial process control, the Ideal Gas Constant serves as the invisible bridge linking pressure, volume, temperature, and the amount of gas in a system—making it foundational to modern physics and engineering. Defined as R = 8.314 J/(mol·K), this universal constant quantifies the amount of energy per mole of gas required to maintain one degree of temperature change at standard pressure. Understanding R is not just about memorizing a number; it's about unlocking the physical principles that govern how gases behave under varying conditions.
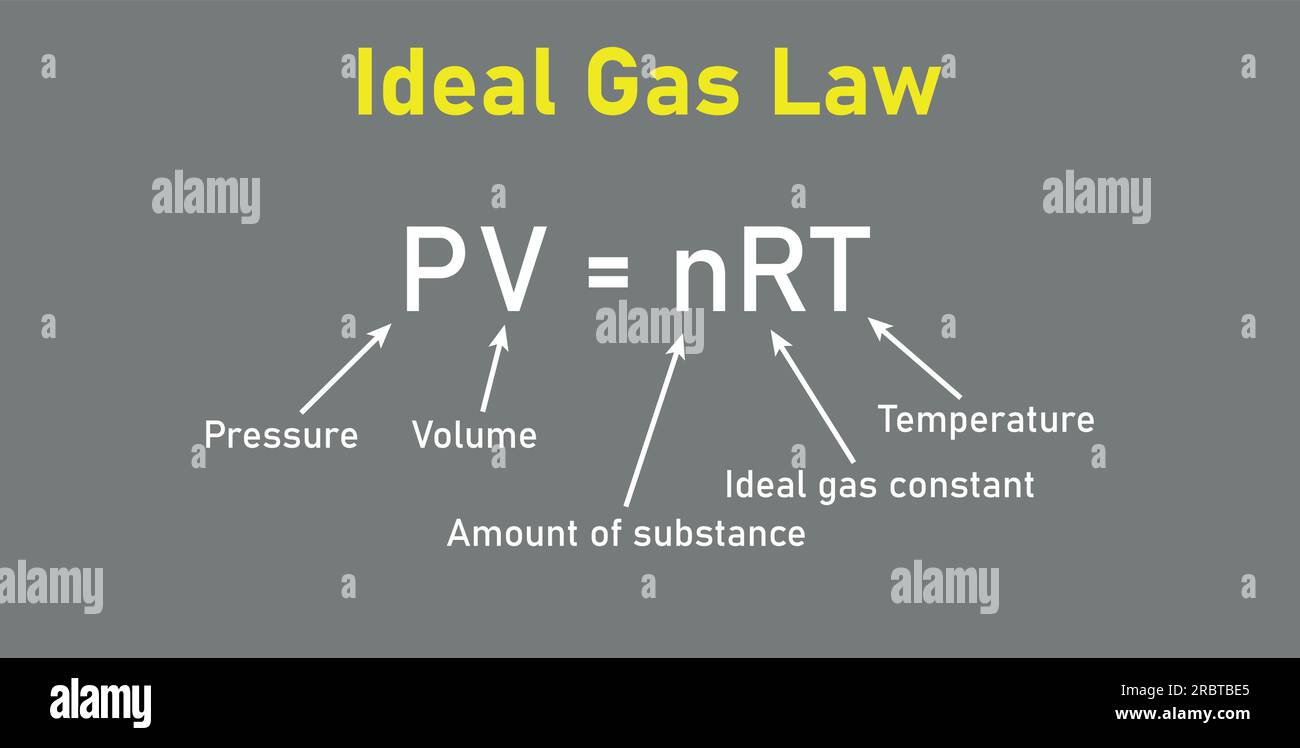
This article explores the Ideal Gas Constant’s role, theoretical underpinnings, real-world applications, and enduring significance in science and industry. Central to thermodynamics, the Ideal Gas Law — PV = nRT — hinges on the constant R to unify gas behavior across physical variables. This equation states that at a fixed temperature, the product of pressure and volume equals the number of moles of gas times R, multiplied by the absolute temperature.
“The Ideal Gas Constant transforms abstract measurements into measurable relationships,” explains Dr. Elena Torres, a senior physicist at the National Center for Applied Thermodynamics. “Without R, we could not compute how temperature changes with pressure in sealed containers or volume expansions in engines.” The concept of R emerged from centuries of experimentation on gases, crystallizing during the 18th and 19th centuries with contributions from scientists including Jacques Charles, Joseph Gay-Lussac, and later, Rudolf Clausius.
Though real gases deviate from ideal behavior under high pressure or low temperature—often described by corrections like the van der Waals equation—R remains the benchmark constant for approximating ideal conditions. Its precision enables engineers and researchers to predict gas behavior with remarkable accuracy in standard environments.
The Role of R in Converting and Comparing Gas Phenomena
In analytical chemistry and process engineering, the Ideal Gas Constant acts as a conversion factor between microscopic particle behavior and macroscopic measurements.For example, when monitoring gas flow in pipelines or designing scuba tanks, engineers use PV = nRT to convert between mass, volume, and temperature. This conversion is essential for safety and efficiency. Consider a natural gas processing plant: measuring a pipeline pressure change requires knowledge of the gas composition and temperature.
By applying PV = nRT with R fixed, operators calculate the exact number of moles present, enabling precise control over combustion, storage, and transport. “R is the constant thread that ties thermal energy to mechanical displacement,” notes Marc Lin, a chemical process engineer, “allowing us to model reactions and optimize energy use.” In educational settings, the Ideal Gas Constant anchors countless laboratory experiments — from demonstrating gas laws with syringes and pressure sensors to simulating atmospheric conditions. Students use it daily to connect theoretical equations with tangible observations, reinforcing an intuitive grasp of molecular kinetics and thermodynamic stability.
Real-World Applications: From Climate Models to Cryogenics
Beyond engineering labs, R powers advancements across scientific disciplines. In meteorology, atmospheric models rely on PV = nRT to simulate how temperature and pressure shifts drive weather patterns. By tracking the ideal gas response of air masses, forecasters predict storm development and climate trends with greater reliability.In cryogenics, where extreme temperatures dominate, R helps scientists calculate phase transitions and gas liquefaction. For example, R is crucial in estimating the operating conditions for liquid nitrogen or oxygen storage—vital for medical and aerospace applications. “Without R, we’d struggle to design systems that maintain stable cryogenic temperatures,” explains Dr.
Lin. “It’s the constant that grounds theory in practice.” Industrial applications extend to HVAC systems, where R enables engineers to size compressors and ducts by estimating load demands based on anticipated gas behavior. Pressure cookers—simple yet powerful devices—also depend on R: increasing internal pressure elevates boiling points, accelerating cooking times safely and efficiently.
In aerospace, R’s predictive capability supports propulsion design, particularly in rocket engines where precise control of fuel vaporization and expansion is critical. By modeling exhaust gas expansion via the ideal gas equation, engineers optimize thrust efficiency and minimize thermal stress on components. This precision directly impacts mission success and fuel economy, underscoring R’s pivotal role beyond terrestrial labs.
The Ideal Gas Constant also enables interdisciplinary innovations.
In environmental science, it supports carbon capture technologies by quantifying how gases diffuse and react at high pressures. In medicine, R aids in ventilator design, helping tailor airflow to patient-specific volumes and temperatures for respiratory support. In education, it remains a cornerstone concept—repeatedly proving indispensable in both foundational instruction and advanced research.
Across scales and disciplines, the Ideal Gas Constant remains a linchpin of scientific understanding.
Def


Related Post

Carpenter Porter Funeral Cremation Services: Honoring Legacy, Supporting Families, and Guiding Obituaries Through 2015 Innovations

Threshold Ap Human Geography: Defining the Edge of Human Activity

Rouba Saadeh and Michele Morrones: Talent, Resilience, and Inspiration Across Two Remarkable Journeys

Kaitlan Collins Bikini: Fashion, Fearlessness, and Influence on CNN’s Visual Language

