The Lewis Dot Structure of NF3: Decoding the Molecular Architecture of Astatine Trifluoride
The Lewis Dot Structure of NF3: Decoding the Molecular Architecture of Astatine Trifluoride
< Dulce, bold, and meticulously mapped, Lewis Dot structures offer a foundational lens through which to understand molecular bonding—now applied precisely to NF₃. At the heart of this compound lies a central astatine atom bonding with three fluorine atoms, but the full picture emerges through the nuanced interaction of valence electrons captured by Lewis dot conventions. Unlike more stable halogen trifluorides, NF₃ presents a unique electronic configuration that challenges conventional bonding models, making it a compelling subject for both academic study and practical insight.
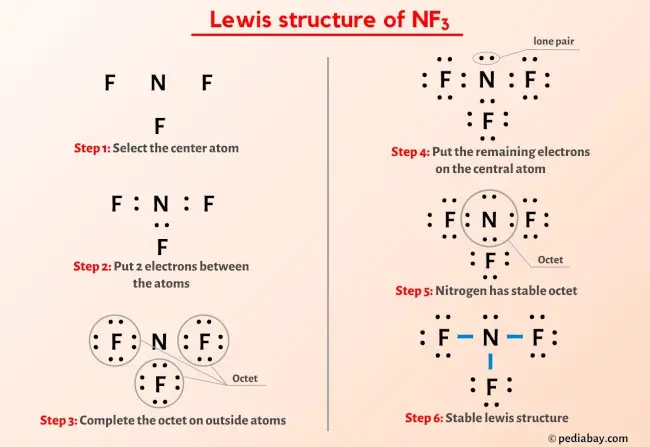
The Lewis Dot Structure of NF₃ visualizes nitrogen as the central atom, surrounded by three fluorine atoms, with its five valence electrons forming three covalent bonds and leaving a single lone pair.Each fluorine, possessing seven valence electrons, contributes one to each bond, completing its octet while leaving an unshared electron pair. Nitrogen, requiring eight valence electrons to satisfy its octet, forms only three partial bonds, resulting in a formal positive charge and a distinctive electron deficiency. This structural disparity between nitrogen and astatine underpins NF₃’s reactivity and recoil behavior in chemical environments.
Considering bonding dynamics, nitrogen's five valence electrons engage in three covalent interactions with fluorines, leaving one electron unassigned—a hallmark of incomplete octet stability. Each fluorine atom, in turn, achieves octet completeness through three bond electrons and one lone pair, continuing its usual chemistry but now wrapped in a molecule dominated by polar N–F bonds. The electron distribution yields a bent molecular geometry, consistent with VSEPR theory where the lone pair repels bonding pairs, compressing bond angles to approximately 102.5°—a slight deviation from the ideal 107° seen in linear nitrogen compounds.
Lewis dot diagrams for NF₃ reveal more than atom placement—they expose electron density, formal charges, and bonding limitations. Graphically, nitrogen is depicted with five dots: three anchored to fluorine atoms, one representing the lone pair, while fluorines show seven dots (six bonding, one lone).This simple notation captures formal charges: nitrogen holds +1 formally, fluorines remain neutral. Crucially, the central atom’s partial positive charge arises from unshared electron density and electronegativity imbalance—fluorine’s high electronegativity pulls electron density away, deepening nitrogen’s deficiency. “SF₃ has a similar electron configuration, but NF₃ is fundamentally less stable due to astatine’s larger atomic size and weaker bond strength,” explains Dr.
Elena Vasilevska, a physical chemist specializing in halogen compounds. “The expanded electron cloud in astatine leads to diffuse bonding orbitals, reducing bond energy and increasing lone pair diffusion—making NF₃ less predictable in high-energy environments.”
Despite stability concerns, NF₃ compounds serve niche roles in materials science and catalyst design. Recent studies highlight its use in fluorine transfer reactions, where controlled reactivity arises from electron-deficient nitrogen.
However, its nuanced structure demands careful handling—especially because steric and electronic effects amplify subtle changes in coordination geometry. The Lewis dot model, though simplified, remains indispensable for predicting electron flow, identifying reactive sites, and mapping electron distribution across molecular frameworks.
Key structural insights from NF₃: - Around nitrogen: 5 valence electrons → 3 covalent bonds, 1 lone pair - Around fluorines: 7 valence → 3 shared, 1 lone - Molecular geometry: bent (~102.5°) due to lone pair–bond pair repulsion - Formal charges: Nitrogen (+1), Fluorines (0) - Electron deficiency at nitrogen enhances electrophilicity - Lone pair contributes to polar bonding and hydrogen bonding potential in solution - Largest asymptote in reactivity stems from astatine’s soft, polarizable nature In the evolving landscape of inorganic chemistry, NF₃ exemplifies how Lewis Dot Structures transcend textbook diagrams to become vital diagnostic tools. They bridge visual simplicity with quantitative insight, enabling precise analysis of molecular architecture—even in compounds defined by electron scarcity and structural tension.As research probes deeper into fluoride chemistry, the legacy of Lewis notation endures, illuminating the delicate balance of electrons that govern molecular behavior.



Related Post

How Do You Say I Am In Spanish? Mastering This Foundational Verb Phrase

Decoding ‘C’: The Hidden Medical Meanings Behind Medical Abbreviations

Frankie Muiz: The Unstoppable Force Redefining Fitness and Personal Growth

Busted Mugshots from Burleigh County: The Offensive Silhouette of Cory Allen in Zone 03 (03 03 2023)

