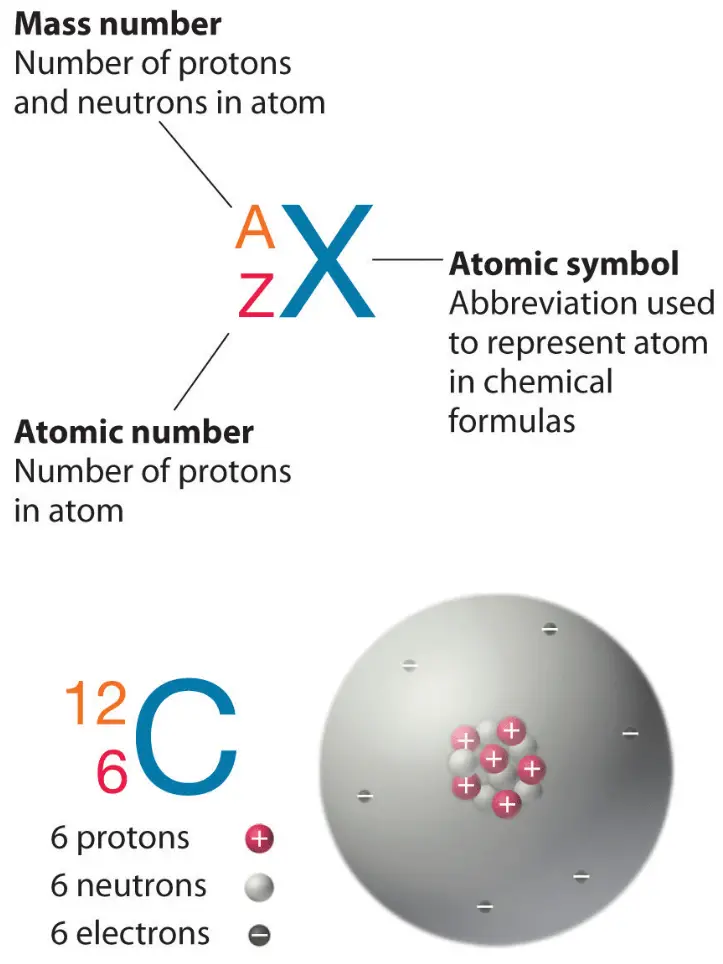
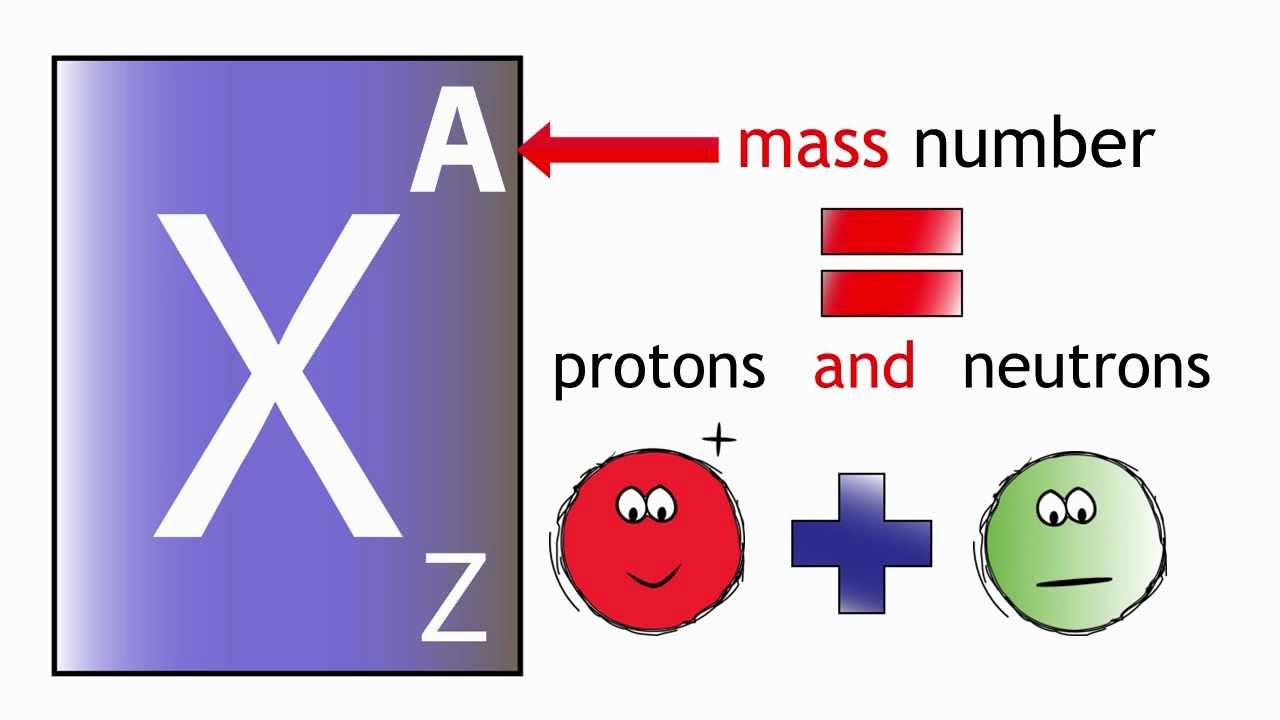
The Nucleon Number of Aluminium: Unlocking Its Atomic Identity
The Nucleon Number of Aluminium: Unlocking Its Atomic Identity
Aluminium, the third most abundant element in Earth’s crust, owes its fundamental atomic structure to its precise nucleon count—comprising protons and neutrons within its nucleus. With an atomic number of 13, aluminium possesses 13 protons, establishing its identity and governs its chemical behavior. Beyond that, the total number of nucleons—protons plus neutrons—defines its isotopic stability and nuclear properties, crucial for applications ranging from aerospace to packaging.
The most common naturally occurring isotope of aluminium is aluminium-27 (²⁷Al), a nucleus formed by 13 protons and 14 neutrons, resulting in a well-balanced, stable configuration that underscores aluminium’s widespread industrial utility. Understanding the nucleon number in aluminium reveals deeper insights into its nuclear and chemical resilience. While aluminium-27 dominates nature’s abundance, minor isotopes such as aluminium-25 (with 12 neutrons) exist naturally but are exceedingly rare, underscoring the special role of the 13-proton core.
A student of nuclear physics recognizes that each nucleon contributes to the strong nuclear force that overcomes electrostatic repulsion, enabling aluminium’s durability in extreme environments. This balanced atomic architecture—13 protons anchored by a stable neutron count—forms the foundation of aluminium’s versatility and reliability across industries.
Thorough examination of aluminium’s isotope composition reveals that though ¹³Al is preferred, other variants like aluminium-26 (¹³ protons, 13 neutrons) are also present in trace amounts, mostly through cosmic ray interactions or stellar nucleosynthesis.
In contrast, isotopes heavier than ¹⁷Al – such as aluminium-28 (¹³ protons, 15 neutrons) – are highly unstable and decay rapidly, making them irrelevant in terrestrial settings. The stable isotopes, especially ²⁷Al, exhibit long half-lives, ensuring their persistence in geological and industrial systems. This isotopic stability arises from the optimal neutron-to-proton ratio, a key determinant of nuclear cohesion.
With 14 neutrons for protons, ²⁷Al strikes a rare but resonant balance that sustains atomic integrity across millennia.
The nucleon count directly influences aluminium’s behavior in chemical reactions and physical transformations. With 13 protons dictating valence electron arrangement and 14 neutrons providing nuclear mass without disruptive imbalance, the atom supports predictable reactivity. Aluminium’s tendency to lose three electrons to form a +3 ion, characteristic of Group 13 elements, stems from its electronic structure—but this process is only meaningful when considering the stable nuclear framework beneath.
“The 13-proton nucleus creates an environment where electron loss manifests predictably, enabling precise material engineering,” notes Dr. Elena Marquez, a nuclear chemist specializing in metalloids. “Without nuclear stability, aluminium’s reactivity would be erratic, undermining its value in manufacturing.”
In industrial applications, the isotopic purity of aluminium subtly affects performance.
While commercial grades often use ²⁷Al due to its natural abundance and inertness, trace differences in neutron counts across isotopes can influence neutron scattering in neutron activation analyses—techniques vital for quality control in aerospace alloys and semiconductor fabrication. Metallurgists rely on consistent isotopic composition to ensure material reliability, particularly in high-stress applications like aircraft components or structural reinforcements. Variability in hydroxide or oxide layers formed on aluminium surfaces can be traced back to isotopic nuances, affecting corrosion resistance and surface bonding.
Advancements in nuclear physics and isotope separation technologies now allow controlled enrichment of aluminium isotopes, opening doors to precision applications. For instance, isotopically enriched ¹³Al-27 is being explored in neutron absorption studies, where predictable nuclear energy levels enhance reactor monitoring and safeguards. “By tailoring nucleon composition, scientists can optimize aluminium for targeted functions—from radiation shielding to quantum computing substrates,” explains Dr.
Rajiv Patel, a materials physicist at the National Institute of Standards and Technology. “This precision elevates aluminium from a commodity metal to a versatile, engineered material.”
The nucleon number of aluminium—centered on 13 protons and 14 neutrons in its most prevalent isotope—epitomizes the synergy between atomic structure and real-world application. It is not merely a number but the silent architect silently determining aluminium’s stability, reactivity, and utility.
From the surface of Earth’s landscapes to the inner workings of advanced technology, aluminium’s identity remains rooted in its defined nucleus—a testament to nature’s precision and humanity’s growing mastery over atomic architecture.
In summary, the nucleon count of aluminium is far more than a technical detail; it defines the element’s atomic identity, influences its nuclear stability, and underpins its industrial dominance. With 13 protons and 14 neutrons in the standard isotope, aluminium emerges as a paradox of simplicity and strength—a metal whose identity rests on invisible forces, yet shapes the visible world across industries, research, and innovation.
Related Post

Roblox Furries: Where Virtual Identity Meets Creative Freedom in a Digital Wild West

Tiffany & Co. At Amsterdam Airport Schiphol: Where Heritage Meets Luxury in Air Travel

Al Pacino’s Financial Empire: How an Iconic Actor Built a Legendary Net Worth Through Talent, Prestige, and Strategic Ventures
Unveiling The Tapestry Of Identity: Exploring Slash Ethnicity

