Unlocking Cellular Precision: How Delta G Rxn Formula Drives Biochemical Equilibrium
Unlocking Cellular Precision: How Delta G Rxn Formula Drives Biochemical Equilibrium
Delta G Rxn Formula stands at the forefront of biochemical justification, offering a precise quantitative lens through which to analyze reaction spontaneity and equilibrium—cornerstones of metabolic pathways, drug design, and cellular energetics. At its core, the formula ΔG = ΔH – TΔS connects thermal energy, enthalpy, and entropy, enabling researchers and clinicians to predict whether a reaction proceeds naturally under physiological conditions. When ΔG is negative, a reaction is thermodynamically favorable; when positive, it requires external input.
This principle, governed by the Delta G Rxn framework, underpins everything from ATP hydrolysis to enzyme catalysis, making it indispensable in both fundamental biochemistry and applied pharmaceuticals.
Understanding the Delta G Rxn Formula begins with unpacking its components: ΔG (Gibbs free energy change), ΔH (enthalpy change), T (absolute temperature in Kelvin), and ΔS (entropy change). Together, these variables form a predictive engine for cellular processes.
ΔH captures the net heat exchange—energy absorbed or released during bond formation and breaking—while ΔS reflects the degree of disorder introduced or reduced. Currently, standard conditions place ΔG° at zero when pressure and concentration match physiological baselines, but real cellular contexts demand appreciation of actual free energy shifts influenced by ion gradients, pH, and macromolecular interactions.
The Thermodynamic Backbone: How ΔG Defines Reaction Fate
Delta G determines whether a reaction unfolds spontaneously or requires intervention. For instance, ATP hydrolysis (-30 to -50 kJ/mol under standard conditions) releases energy because the products exhibit lower free energy, driving processes like muscle contraction and active transport.Yet, in vivo, actual ΔG often deviates from ΔG° due to concentration gradients and compartmentalization—factors critical in metabolic flux regulation. The Delta G Rxn Formula thus serves not as a rigid rule but as a calibrated guide, adapting to biological nuance. Different reaction types influence ΔG differently: - Exergonic reactions - Endergonic reactions 0): Energy-consuming, non-spontaneous conversions requiring coupling with exergonic steps, such as biosynthesis or active transport. The coupling mechanism—exemplified by ATP-lysine ligase or Na⁺/K⁺ pumps—relies on ΔG principles to ensure directional control and thermodynamic coherence across cellular networks. Real-world application hinges on accurate ΔH and ΔS estimation, even when variables soar beyond standard thermodynamic tables. Enthalpy is typically measured via calorimetry, capturing bond energetics during bond cleavage. Entropy, though more elusive, often increases with reactant disorder or solvent reorganization—especially crucial in aqueous environments where hydration shells shift dynamically. Combining these with temperature in the ΔG = ΔH – TΔS equation yields the net free energy driving biological change. Consider glycogen synthesis: endergonic, yet powered by ATP hydrolysis—a classic example of energy coupling. Here, ΔG° for the biosynthetic step may be positive (+, indicating unfavorability), but when ATP hydrolysis (-ΔG0’ ~ -30 kJ/mol) supplies the latent energy, the total ΔG shifts decisively negative, making the reaction viable. This energetic headroom enables the cell to "bank" chemical energy, selectively activating pathways only when Free Energy (ΔG) permits. Another pivotal example lies in ion gradient maintenance. The sodium-potassium pump consumes ATP to transport Na⁺ out and K⁺ in, imposing an unfavorable ΔG overall. Yet without this active work, electrophysiological signaling and secondary active transport would collapse. The Δ G Rxn Framework thus reveals how thermodynamic constraints sculpt cellular architecture and communication.Quantifying Spontaneity Across Biological Systems
Biological systems harness ΔG Rxn insights to translate thermodynamics into function.Clinical and Pharmaceutical Implications
In drug development, the Delta G Rxn Formula guides rational design by identifying metabolically feasible targets. Medicinal chemists predict how small molecules interact with enzymes by assessing ΔG of binding—energetically favorable interactions are more likely to yield viable therapeutics. For instance, inhibitors designed to stabilize an enzyme’s inactive conformation must reduce the free energy of the complex, effectively lowering ΔG for binding.
Conversely, activators aim to enhance substrate affinity by stabilizing transition states. Moreover, pharmacokinetics depends on ΔG’s influence on drug absorption and distribution: lipid-soluble compounds with favorable ΔG for crossing membranes pass efficiently through cell barriers. In oncology, targeting metabolic vulnerabilities—such as cancer cells’ reliance on Warburg effect glycolysis—relies on manipulating ΔG dynamics to tilt energy balance irreversibly toward apoptosis.
Thus, Delta G Rxn is not confined to lab benches but shapes real-world therapies.
The Future of Biochemical Prediction in a Systems View
As computational biochemistry evolves, the Delta G Rxn Formula gains refined precision through molecular dynamics simulations and high-throughput thermodynamic profiling. These advances allow integration of contextual variables—pH, competing ions, post-translational modifications—into ΔG calculations previously grounded in idealized conditions.Machine learning tools now parse vast datasets to predict ΔG trends across cellular networks, accelerating drug discovery and personalized medicine. Yet fundamental understanding remains essential: thermodynamics dictates possibility, but biological context defines performance. The Delta G Rxn Formula is both a molecular magnifying glass and a predictive compass, ensuring every biochemical innovation respects nature’s energetic laws.
In essence, Delta G Rxn is far more than an equation—it is the language of life’s energy. By decoding reaction spontaneity through ΔH, T, and ΔS, scientists illuminate pathways of metabolism, design smarter therapeutics, and decode the language of cellular decision-making. As research deepens, this thermodynamic cornerstone ensures that biochemical progress remains not just visionary, but grounded in irrefutable physics—one free energy calculation at a time.


Related Post

Decoding Curve Finance’s AMM Formula: How Uniswap’s Hidden Math Powers DeFi’s Liquidity Revolution

The Science Behind Production: Unlocking Producer Science Definition for Modern Excellence

Times Square, New York: Your Zip Code and Ultimate Area Guide Dropping Live Big Smoke
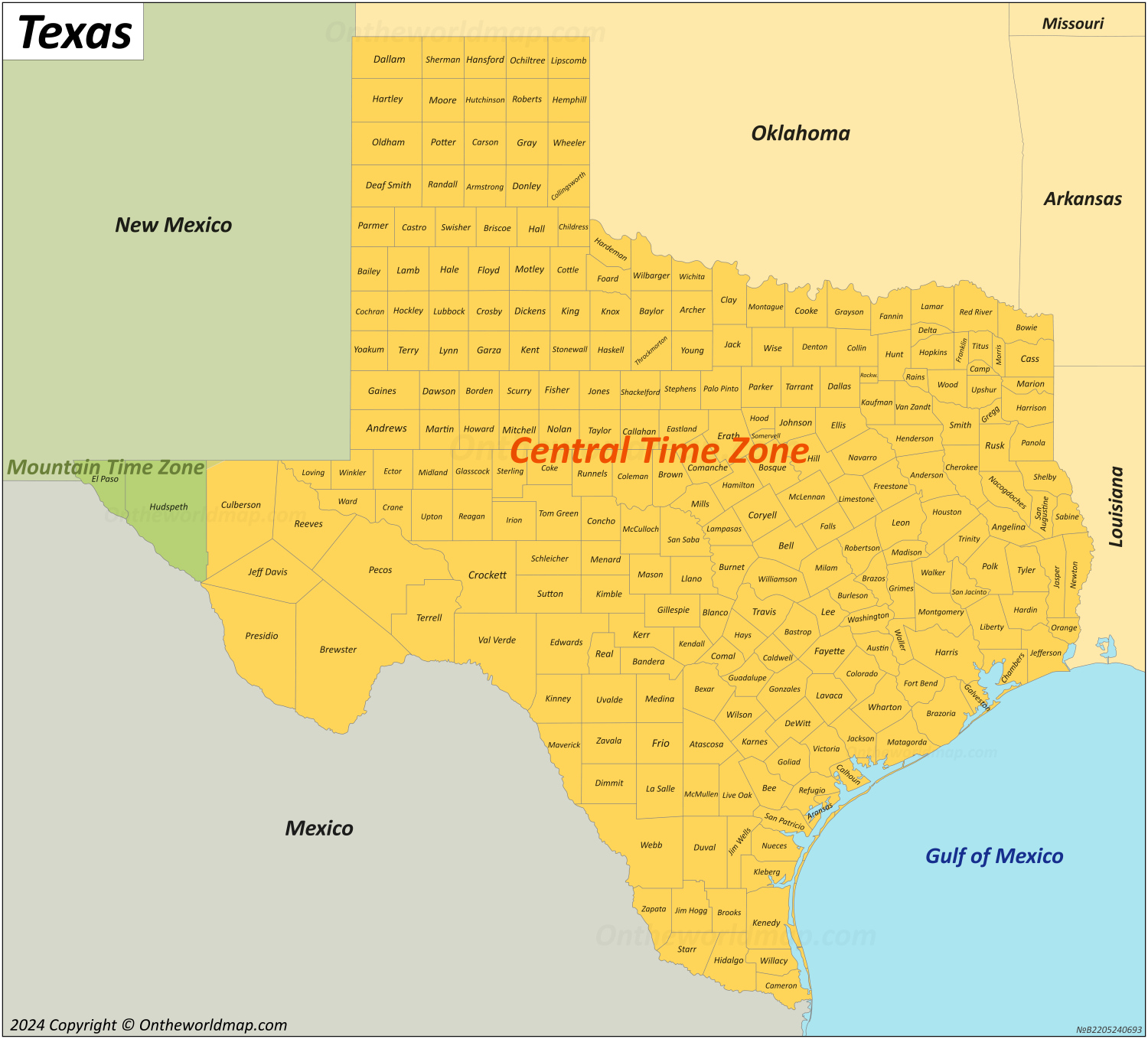
Time Zone Dallas Texas: How a Central Time Heartbeat Shapes Daily Life and Business

