Unlocking Energy Reactions: How Ionisation Enthalpy Powers Chemistry’s Most Critical Processes
Unlocking Energy Reactions: How Ionisation Enthalpy Powers Chemistry’s Most Critical Processes
Ionisation enthalpy—the energy required to remove an electron from a gaseous atom—stands at the heart of understanding chemical reactivity, stability, and bonding. Far more than a mere thermodynamic parameter, it serves as a fundamental lens through which scientists decode how atoms interact, bonds form or break, and materials transform under energy forcing. As both a cornerstone of atomic physics and a practical tool in chemical kinetics, ionisation enthalpy bridges theory and application across diverse fields—from atmospheric science to pharmaceutical design.
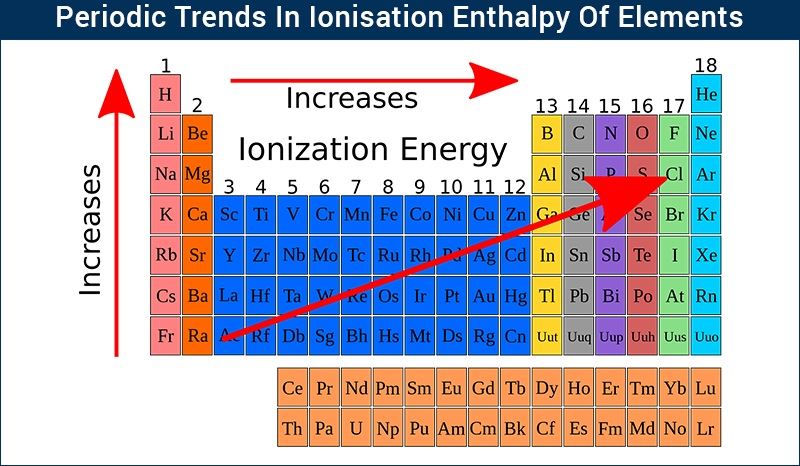
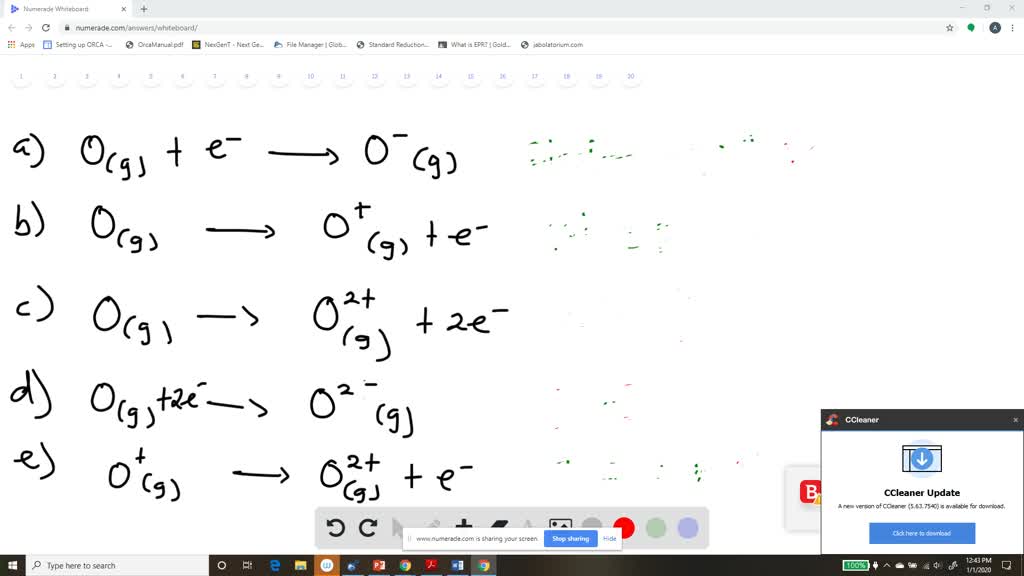
The principle of ionisation enthalpy rests on the fundamental energy needed to withdraw an electron from a neutral atom in its gaseous state, shifting it to a positively charged ion (A → A⁺ + e⁻). This process reveals atomic structure and electron binding strength, expressed in kilojoules per mole (kJ/mol). Lower ionisation energies signal atoms eager to lose electrons, typically alkali metals like lithium and sodium, while high values define noble gases and tightly bound transition metals such as iron or gold.
Measuring ionisation enthalpy involves expatriate techniques under controlled conditions: X-ray photoelectron spectroscopy (XPS) and photoionization spectroscopy are standard methods, offering atomic precision. Each measurement reflects not only an atom’s intrinsic electron affinity but also the subtle influences of electronic shielding, nuclear charge, and orbital symmetry. “Ionisation enthalpy is not a simple number,” explains Dr.
Elena Marquez, a physical chemist at ETH Zurich. “It captures the dynamic interplay of quantum forces—how closely electrons are held by the nucleus and how they shield each other.”
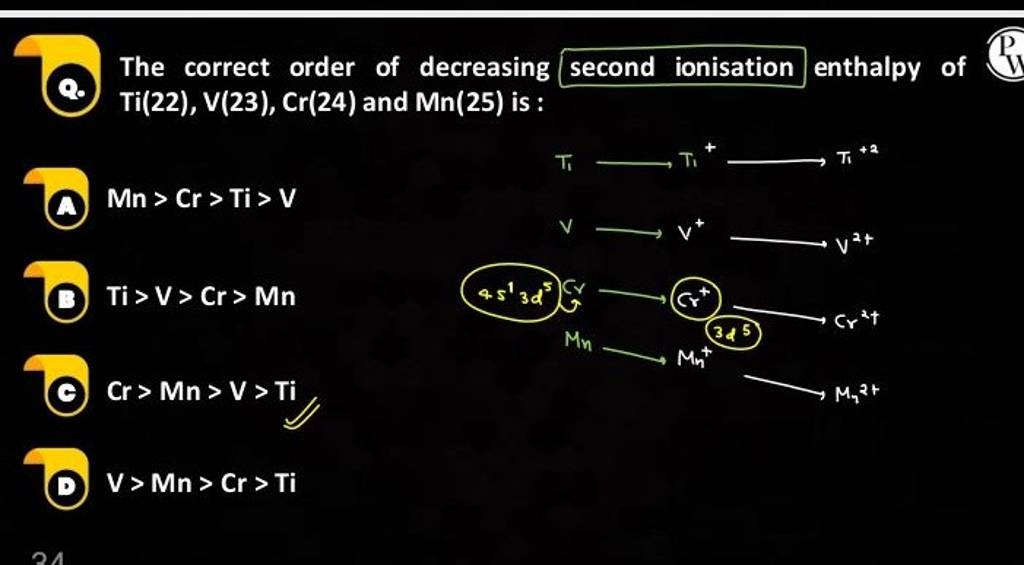
Each successive ionisation reveals a distinct energetic signature. The first ionisation enthalpy (IE₁) measures the initial removal, but IE₂, IE₃, and beyond expose deeper electron shells and quantum effects.
For instance, removing electrons from inner shells—like the 2s or 2p orbitals—requires dramatically more energy than those in outer shells. This stepwise increase explains why beryllium (BE) and boron (B) show progressively higher ionisation energies, reflecting their compact electron configurations and strong effective nuclear charge. In contrast, alkali metals like cesium (Cs) display steeply declining IE values, as their outer electrons reside in diffuse s-orbitals weakly bound to a diffuse, uniformly charged nucleus.
Quantitative variation across the periodic table illustrates ionisation enthalpy’s predictive power.
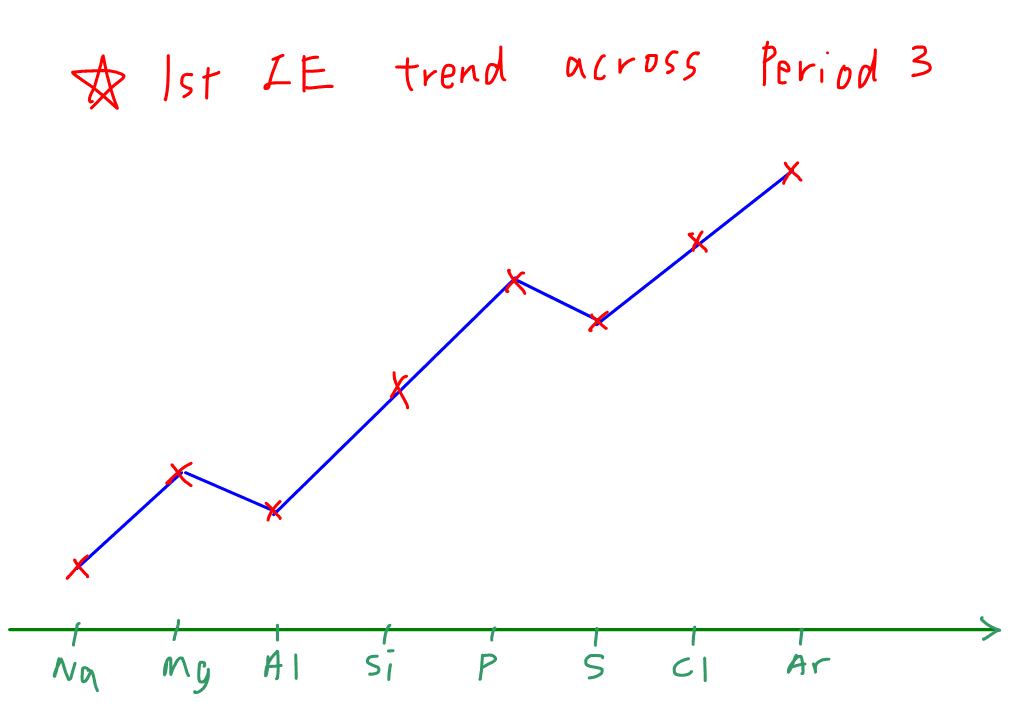
Periods show rising ionisation trends from left to right—driven by increasing effective nuclear charge across a fixed shell—while the periodic table’s groups highlight descending ionisation energies due to shielding and orbital expansion. Transition metals display irregularities: chromium and copper resist efficient electron removal owing to filled or half-filled d-subshells, showcasing how electron configuration and pairing energies influence enthalpy values.
Beyond elemental descriptor, ionisation enthalpy governs reaction energetics. In combustion, ionisation events—often coupled with electron capture in excited states—facilitate rapid bond rearrangements, liberating energy.
For example, during the oxidation of methane (CH₄), electron transfer processes involving ionisation influence reaction pathways and activation barriers. Likewise, in plasma chemistry, the energy required to ionise gas-phase molecules dictates ionization rates, crucial for fusion reactors
Related Post
Secure Boot & American Megatrends (AMI) BIOS: Fortifying Firmware from the Ground Up

How to Be Rich in GTA 5: Master Wealth Through Strategy, Not Luck

Saginaw Bay Fishing Report: Where Every Cast Uncovers a Freshwater Treasure
Ist Es Over Fur Mich? The Surprising Truth Behind This Debate

