Unlocking Matter's Permanence: How the Law of Constant Composition Defines Chemical Identity
Unlocking Matter's Permanence: How the Law of Constant Composition Defines Chemical Identity
The perpetual dance of atoms within compounds reveals a fundamental truth: while elements rearrange, the proportions they maintain remain unshakable. Governed by the Law of Constant Composition, a cornerstone of chemistry, this principle asserts that a pure chemical compound always contains the same relative amounts of constituent elements—no exceptions, no variability. This immutable ratio not only anchors the identity of substances but also enables precise chemistry, from pharmaceuticals to industrial synthesis.
Understanding this law unlocks deeper insight into why water boils at exactly 100°C (when composed as H₂O in fixed proportions) and why iron continues to rust at the same stoichiometric rate under consistent environmental conditions.
At its core, the Law of Constant Composition—also known as Proust’s Law after French chemist Joseph Proust, who robustly defended it in the late 18th century—states that a given compound formed under consistent conditions will consistently exhibit fixed elemental ratios. For instance, water consistently combines two hydrogen atoms with one oxygen atom by mass, regardless of source—be it rainfall, glass, or fusion in a star.
This consistency enables both reproducibility and trust in scientific and industrial processes.
The Origins and Formation of the Law
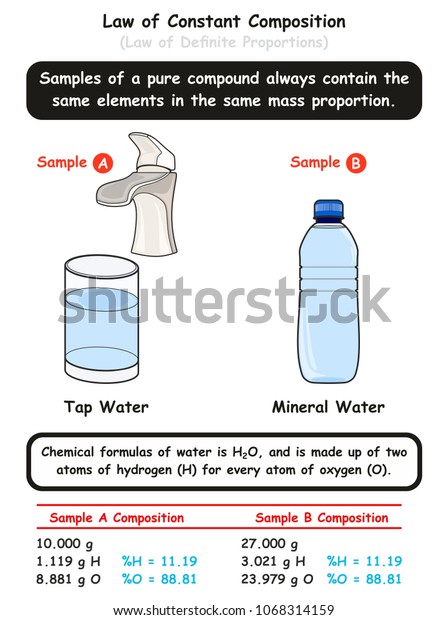
The idea that matter maintains fixed proportions in compounds emerged not from mystical insight but from rigorous experimentation. Joseph Proust challenged earlier notions, such as those of the alchemists and early chemists who believed mixtures varied elementally. In systematic trials, Proust repeatedly synthesized water from hydrogen and oxygen gas, measuring outputs meticulously.He observed that no matter the batch, water always contained approximately 11.19% hydrogen by mass and 88.81% oxygen—equivalent to the mass ratio of 1 to 8. This empirical evidence proved that composition, once defined, remains immutable.
Later, Amedeo Avogadro’s hypothesis that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules provided the theoretical foundation.
Combined with Proust’s empirical results, the law solidified, enabling Avogadro’s number to become the universal bridge between mass, volume, and molecular count in stoichiometric calculations.
The Role of the Law in Modern Chemistry
Today, the Law of Constant Composition underpins nearly every chemical process. It validates the mole concept—a cornerstone of quantitative analysis—and ensures that reactions obey predictable, repeatable ratios. Consider the synthesis of ammonia in the Haber process: nitrogen and hydrogen combine in a fixed 1:3 ratio (N₂:H₂), producing ammonia (NH₃) with a consistent elemental composition.Any deviation in input proportions—deviations that violate the law—results in incomplete reactions and substandard yields.
Beyond industrial applications, this principle ensures reliability in scientific research. In pharmaceutical manufacturing, every dosage must rely on compounds with precisely known stoichiometry; otherwise, efficacy and safety collapse.
Similarly, forensic analysis depends on elemental ratios to trace materials back to their sources—whether oils, minerals, or synthetic substances—all anchored by the immutable truth of constant composition.
Examples Showcasing the Law’s Universality
Take water (H₂O): regardless of whether it falls as snow, flows in a river, or is distilled in a lab, its molecular makeup is unchanging. A single molecule contains two hydrogen atoms and one oxygen atom, defining a 2:1 elemental ratio by number and a 1:8 mass ratio.This consistency explains why boiling point, density, and chemical behavior define water uniquely—no variation permitted by composition.
In iron oxidation, the transformation of rust follows the same rules. Iron reacts with oxygen to form iron(III) oxide (Fe₂O₃), always with a fixed 2:3 ratio between iron and oxygen by atoms.
This predictable outcome enables corrosion modeling and protective coating development, where understanding the exact composition of oxide layers guides material design.
Exceptions and Misconceptions Clarified
Despite its certainty, the Law of Constant Composition applies strictly within the bounds of well-defined compounds under consistent conditions. Deviations arise only under non-standard circumstances—extreme temperatures, pressures, or impurities—that disrupt equilibrium.For example, aqueous solutions of copper sulfate (CuSO₄·5H₂O) strictly maintain 68.5% copper, 10.5% sulfur, and 21% oxygen by mass. If contaminated with other ions or exposed to dehydration, the exact ratio changes—violating the fundamental principle.
Another common confusion arises when distinguishing elemental composition from molecular formula.
While H₂O’s formula indicates two hydrogen atoms per oxygen, the ratio by mass reflects actual atomic masses: hydrogen averages 1 g/mol, oxygen 16 g/mol, yielding a 2:16 = 1:8 mass ratio. This distinction highlights that the Law protects not just how atoms are named, but precisely how they combine in quantified form.
The Enduring Legacy of Proust’s Insight
The Law of Constant Composition stands not merely as a historical footnote but as a living principle empowering science and industry.From lab benches to production lines, its unwavering truth ensures consistency across chemical processes, fostering innovation grounded in reliability. As researchers push boundaries in materials science and environmental chemistry, the law remains their constant: a truth written in the immutable language of atoms. In a world where change is constant, the Law of Constant Composition offers stable anchors—verifying substance, enabling discovery, and defining what it truly means for matter to remain itself.


:max_bytes(150000):strip_icc()/scientist-holding-a-molecular-model-of-a-chemical-formula-556421537-5762c3715f9b58f22edbf3d2.jpg)
Related Post

Chronic Chaos at Newark: The Unrelenting Toll of Airport Delays on Travelers

High Desert State Prison A Comprehensive Guide: Safety, Structure, and Systems Behind One of Oregon’s Most Strategically Located Correctional Facilities

Discover Your Home in Wilmington, NC—Find Your Perfect Zip Code

Glamping Jackson Hole: Where Luxury Meets the Wild

