Unlocking the Secrets of Sulfur Hexafluoride: Precision in Lewis Structure Reveals Key to Its Chemistry
Unlocking the Secrets of Sulfur Hexafluoride: Precision in Lewis Structure Reveals Key to Its Chemistry
Sulfur hexafluoride (SF₆), a highly symmetrical and thermally stable compound, stands as one of the cornerstone materials in industrial and scientific applications—from gas insulation in high-voltage electrical equipment to use in semiconductor manufacturing. At the heart of understanding its remarkable properties lies its detailed Lewis structure, a fundamental representation that demystifies electron distribution and bonding behavior. The Lewis structure of SF₆ not only clarifies molecular geometry but also reveals insights into its inertness, polarizability, and strong fluoroalkyl character, making it indispensable in both classroom chemistry and cutting-edge research.
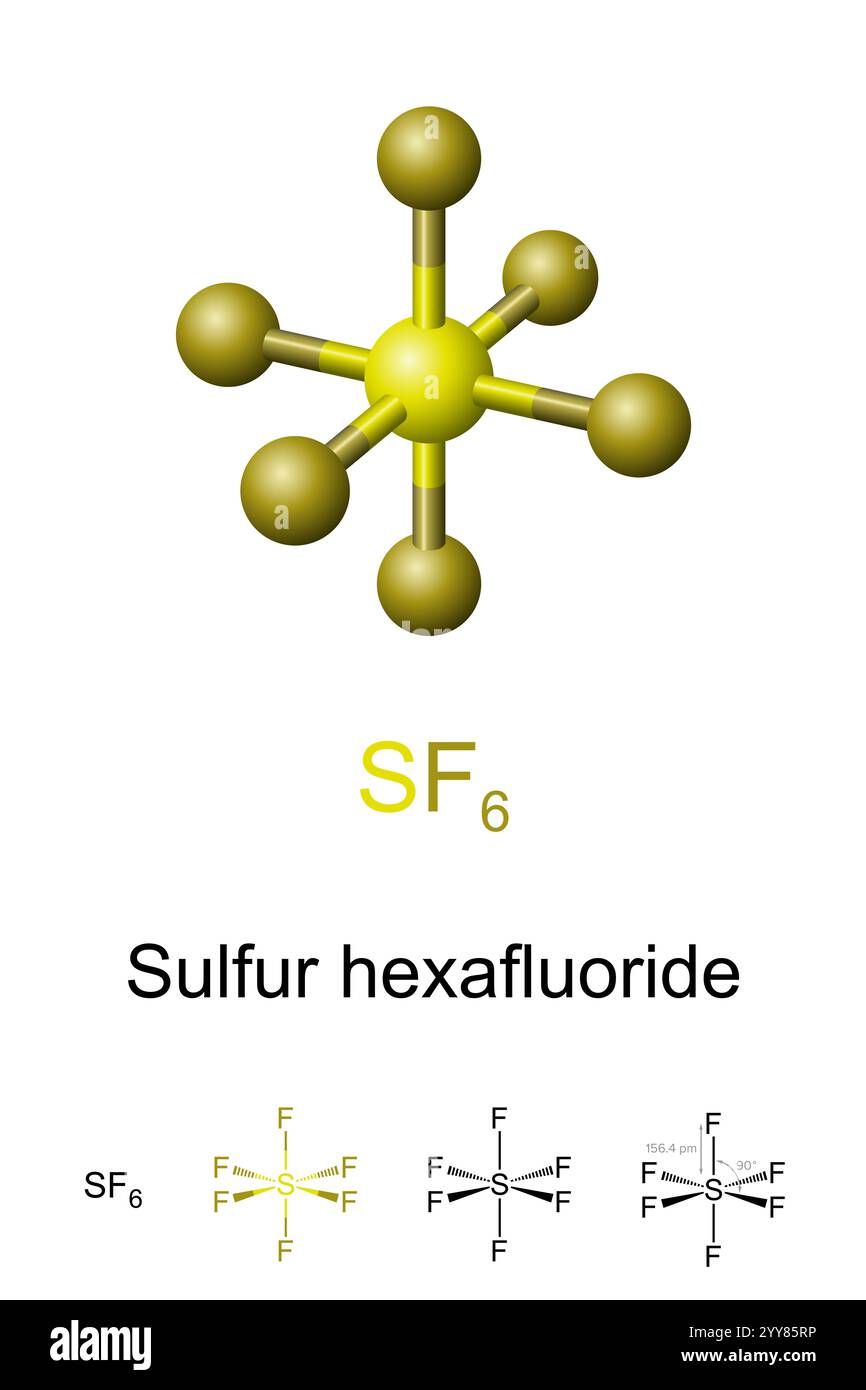
At first glance, SF₆ appears deceptively simple: one sulfur atom centrally bonded to six fluorine atoms in an octahedral arrangement.
Yet, its Lewis structure exposes far more than just VSEPR predictions. By analyzing valence electron allocation and formal charge, chemists gain precise insight into molecular stability and reactivity. Unlike many sulfur compounds that readily participate in redox reactions, SF₆ is unusually robust, a trait deeply rooted in its electron configuration and bond geometry.
The Core Architecture: Sulfur Hexafluoride’s Electron-Pair Architecture
Central to SF₆’s stability is its octahedral geometry, derived from six bonding electron pairs surrounding sulfur with no lone pairs.
The sulfur atom, in the +6 oxidation state, shares six valence electrons—one with each fluorine atom—forming strong S–F bonds characterized by significant electronegativity difference and directional sp³d² hybridization. This hybridization allows optimal overlap of sulfur’s d-orbitals with fluorine’s p-orbitals, enabling efficient bonding across 90-degree axes in three-dimensional space. The result is a molecule so symmetric that dipole moments cancel completely, rendering SF₆ nonpolar despite polar bonds.
The octahedral framework of SF₆ is not merely a geometric curiosity—it directly governs the compound’s industrial utility.
Industries rely on its chemical inertness, thermal resistance, and dielectric strength, all of which stem from balanced electron distribution. For instance, SF₆’s inability to diffuse through metal lattices or react with electrode surfaces ensures long operational lifetimes in circuit breakers and transformers. According to Dr.
Elena Torres, a structural chemist at the National Institute of Materials Science, “The precision of the Lewis structure reveals why SF₆ remains chemically ‘cemeterially frozen’—a frozen state that enhances both safety and reliability in high-stakes environments.”
Lewis Structure: Mapping Elements, Bonds, and Formal Charges
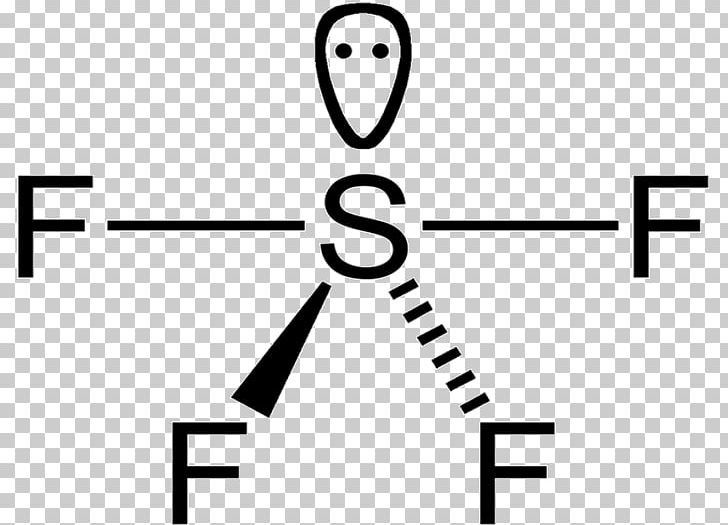
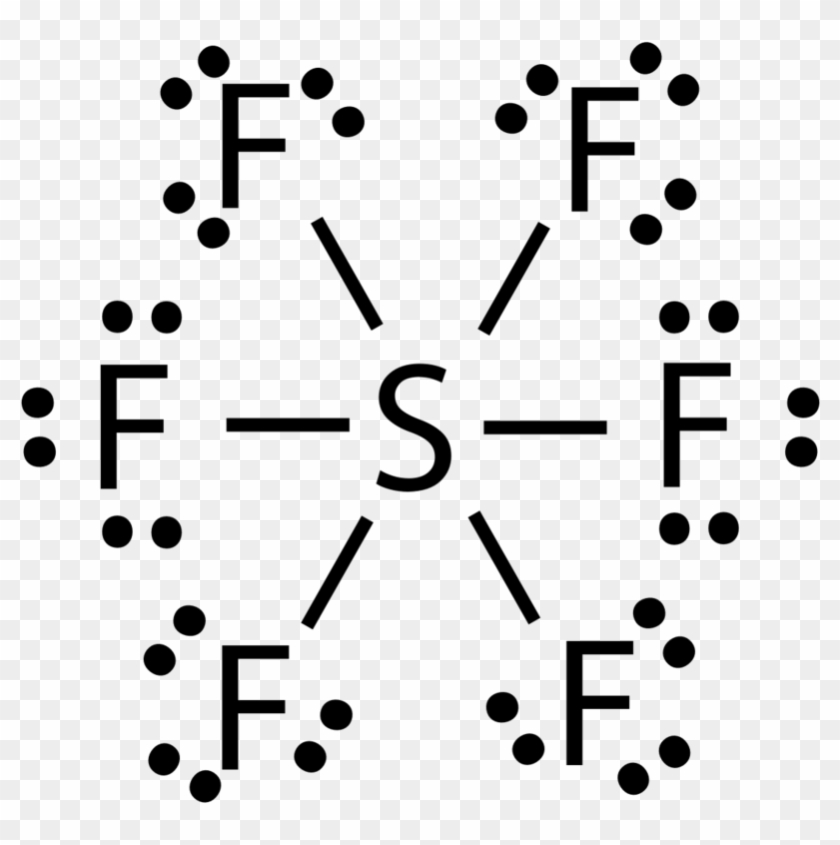
Constructing the Lewis structure of SF₆ begins with counting total valence electrons: sulfur contributes six, and six fluorines contribute 24 electrons (each with seven), totaling 30. These electrons form six equal S–F covalent bonds, using six electron pairs and consuming all available valence pairs. Each fluorine retains three lone pairs, contributing to the molecule’s 3D harmony.
Because sulfur donates one electron to each fluorine and occupies no hybrid orbitals containing lone pairs, all atoms achieve formal charge zero—a hallmark of chemically stable, resonance-free structures.
The absence of formal charge reveals SF₆’s electronic perfection. Unlike molecules with charge separation or radicals, SF₆ presents a flawless electron count: sulfur’s six electrons paired perfectly with fluorine’s lone pairs. This balance minimizes reactivity under ambient conditions and shields the molecule from external degradation pathways.
Even under high temperatures, where molecular vibrations intensify, the electron distribution remains intact—validated by spectroscopic data over the past decades.
Electronegativity and Bonding Strength in SF₆
The striking bond stability arises from the electronegativity contrast: fluorine (3.98) pulls electron density toward itself, creating strong dipole-active bonds. Sulfur’s (+6) oxidation state amplifies bond polarity, enhancing lattice cohesion when aggregated into solid dielectric forms. Crucially, the ligand field symmetry around sulfur allows minimal electron delocalization and prevents homolytic cleavage—an essential feature for long-term dielectric performance in electric infrastructure.
Industrially, SF₆’s Lewis structure underpins its unmatched performance in electrical insulation and semiconductor processing.
In gas-insulated switchgear, its high dielectric strength—up to 2.5 times that of air—prevents arcing at elevated voltages. Its chemical density and thermal resilience also support plasma etching during microchip fabrication, where contamination must be avoided at atomic scales. “SF₆’s very structure makes it irreplaceable for decades,” notes Dr.
Marcus Lin, processes engineer at a leading semiconductor firm. “Its bonding architecture ensures no degradation over operational lifetimes, even under extreme thermal cycling.”
Environmental Considerations and the Search for Alternatives
While SF₆’s chemical inertness is a strength at applications, it poses environmental challenges. As a potent greenhouse gas with 23,500 times the global warming potential of CO₂ over a century, its use is tightly regulated.
This has spurred intense research into fluorine-free alternative gases—such as dry air and nitrogen fluoridation schemes—aimed at replicating SF₆’s dielectric performance without long atmospheric lifetimes. Yet, the S₆F₆ Lewis structure remains a benchmark; few compounds match its combination of stability, electron symmetry, and industrial compatibility. Until viable replacements emerge, SF₆’s structure offers both a challenge and a model for designing next-gen electrical fluids.
In essence, the Lewis structure of sulfur hexafluoride is more than a classroom diagram—it is a window into a molecule engineered by nature for unmatched stability and function.
Its octahedral perfection reveals why SF₆ has remained indispensable across energy, electronics, and industrial chemistry, even as scientists seek greener substitutes. Mastery of its electron architecture not only deepens scientific understanding but guides the next evolution of high-performance materials.
Related Post

Josh Radnor: From Indie Breakthrough to TV Stardom – The Career That Redefined an Actor’s Trajectory

Puff Daddy & Biggie Smalls: The Dynamic Duo Who Revolutionized Hip-Hop

What Does "Mia Mia" Mean? Decoding the Expressive Gesture Behind the Popular Phrase

Patricia Rahman: A Starlit Journey Through Iconic Movies and TV Shows