Unveiling the Molecular Dance: Equation Dissociation of Propanoic Acid in Water
Unveiling the Molecular Dance: Equation Dissociation of Propanoic Acid in Water
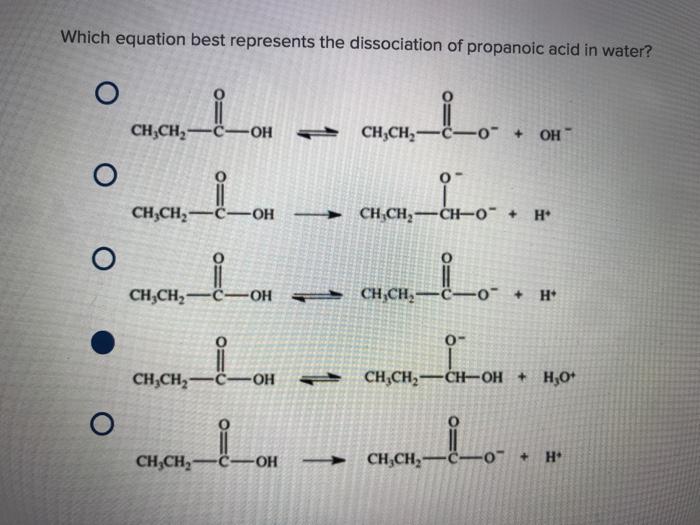
The quiet flux of propanoic acid dissolving in water reveals a fundamental chemical ballet — one that shapes not only industrial processes but also biological systems and environmental chemistry. At the heart of this transformation lies the equation governing its dissociation:
Understanding how propanoic acid behaves in water offers insight into its reactivity, solubility, and role in both laboratory and natural systems. Propanoic acid, a three-carbon carboxylic acid with the molecular formula CH₃CH₂COOH, enters water through a delicate process governed by thermodynamics and intermolecular forces. When introduced, individual acid molecules begin to interact with polar water dwellers—oxygen atoms from water forming hydrogen bonds with the acidic hydrogen and the carboxylate oxygen.
This solvation stabilizes the dissociation step:
For propanoic acid, this yields a modest
Molecular Mechanics: Solvent Interactions and Ion Stabilization Water molecules surround dissociated ions in a dynamic shell, significantly affecting reaction kinetics. The positively charged H⁺ rapidly associates with water ligands to form hydronium ions (H₃O⁺), minimizing proton alteration of neighboring molecules. The conjugate base (A⁻), a negatively charged COO⁻ group, remains solvated by hydrogen-bonded water networks—this stabilization lowers the energy barrier for dissociation.
This solvent-mediated stabilization is critical: without it, the charged species would recombine quickly, suppressing dissociation. Environmental and Industrial Roles In natural waters, propanoic acid contributes to organic carbon turnover and participates in acid-base equilibria affecting aquatic life. Its partial dissociation ensures dynamic pH buffering, vital for maintaining homeostasis in freshwater ecosystems.
In industry, understanding its dissociation is essential for fermentation kinetics (e.g., in propionic acid production by bacteria) and in fertilizer formulation, where solubility and nutrient availability depend directly on proton dynamics in aqueous media. Analytical Insight: Measuring Dissociation in Real Systems Laboratory detection of dissociation relies on pH monitoring, titration, and spectroscopic methods. For instance, potentiometric titration reveals the equivalence point where [H⁺] = [A⁻], confirming partial ionization.
Rapid advances in ion-selective electrodes and NMR spectroscopy allow real-time tracking of equilibrium shifts, offering chemists precise control in process optimization. “Propanoic acid’s dissociation in water isn’t dramatic, but its subtle ion behavior is morally critical—quietly anchoring chemical stability,” notes Dr. Elena Torres, organic chemist at the International Center for Aqueous Chemistry.
Despite its relatively weak dissociation, the behavior of propanoic acid in water exemplifies the nuanced dance between neutral molecules and charged species in aqueous chemistry. Each proton released marks a step in a network of interactions shaped by solvation, thermodynamics, and molecular architecture. Far from inert, this equilibrium underpins applications ranging from pharmaceuticals to wastewater treatment, proving that even faint molecular signals carry profound chemical weight.
Understanding the equation dissociation of propanoic acid in water is not merely an academic exercise—it is a gateway to mastering the invisible forces that govern aqueous chemistry across science, industry, and nature.


Related Post

When Did 9/11 Happen — The Shocking Timeline of a Defining Tragedy

See Your Full PayPal Credit Card Number: What It Means and How to Access It

Surgical Tech Salary In NYC Your Complete Guide: Earnings, Demand, and Career Path

Unlocking Jo In: The Transformative Power of Japanese Innovation in Global Markets

