Unveiling the Secret: The Thiocyanate Lewis Structure and Its Role in Coordination Chemistry
Unveiling the Secret: The Thiocyanate Lewis Structure and Its Role in Coordination Chemistry
The Thiocyanate ion, represented as SCN⁻, occupies a pivotal position in modern inorganic chemistry due to its unique ability to act as a versatile ligand in coordination complexes. Its Lewis structure reveals a fundamental insight into how electron-pair donation shapes molecular behavior, offering both industrial and biological significance. Understanding SCN⁻’s bonding architecture not only clarifies its reactivity but also illuminates broader principles governing transition metal coordination.
Far more than a simple anionic species, thiocyanate’s structural nuances drive applications across catalysis, materials science, and biochemical systems.
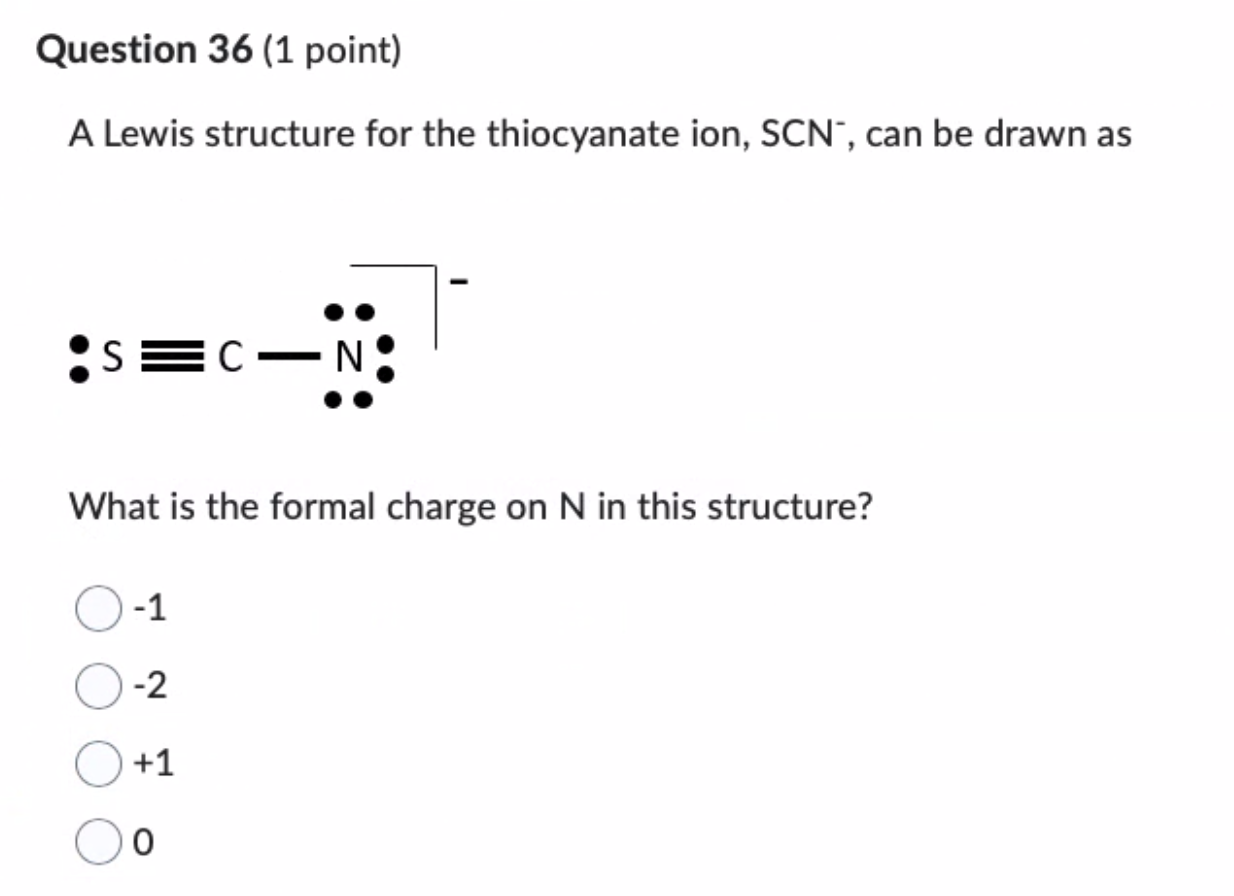
The Lewis structure of thiocyanate is a masterclass in asymmetric bonding, featuring a pivotally significant lone pair donation that defines its role as a monodentate ligand. Central to this structure is the sulfur atom, which bears a lone pair available for donation, while the nitrogen and carbon atoms reside in conjugated p-orbitals that stabilize the ion’s geometry. Unlike rigid molecular frameworks, SCN⁻ adopts a flexible arrangement where lone pair availability dictates reactivity.
The negative charge resides evenly across nitrogen and sulfur, enabling nuanced interactions with metal centers. This electron distribution—often depicted with curved arrows in schematic diagrams—embodies the essence of Lewis acid-base interaction, where electron pair transfer governs complex formation.
Core Atoms and Electron Distribution: The Sulfur Nexus
At the core of thiocyanate’s Lewis structure lies sulfur, the atom responsible for the ion’s structural stability and reactivity. With six valence electrons, sulfur’s industrial relevance extends beyond this anion; it forms part of diverse thiourea, thiol, and metal-thiocyanate complexes.
In SCN⁻, sulfur’s lone pair—resonance-stabilized across the N–S–C framework—acts as the primary electron donor. The charge is delocalized, with sulfur bearing a partial negative charge that enhances its nucleophilic character. Computational studies confirm sulfur’s role as the principal site for coordination, particularly with transition metals such as copper, nickel, and cobalt.
Nitrogen and Carbon: Orchestrating Interaction Through Hybridization
While sulfur captures the dominant share of electron donation, nitrogen and carbon contribute essential structural and electronic balance.
Nitrogen, sp²-hybridized in resonance structures, stabilizes conjugation and strengthens the N–S bond through π-backdonation. Carbon, though less electronegative, provides a rigid linker between nitrogen and sulfur, ensuring geometric stability. This triatomic arrangement—S–N–C—forms the backbone of SCN⁻, with nitrogen’s lone pair reinforcing coordination potential, especially in six-coordinate metal complexes.
The hybridization states directly influence bond lengths and strengths, with literature data indicating C–S distances averaging 1.76 Å and N–S at 1.96 Å.
Resonance and Charge Delocalization: Beyond Simple Dragging
Scrutinizing the Lewis structure through resonance reveals a dynamic equilibrium between two canonical forms: SCN⁻ and [SC(NH₂)₂]²⁺ (or analogous amidine derivatives). While the protonated and amidine forms are empirically observed, SCN⁻ itself is best described as a resonance hybrid, with electron density distributed across both N and S atoms. This delocalization stabilizes the ion, reducing charge localization and allowing graceful adaptation to diverse coordination environments.
The resonance effect explains why thiocyanate can act as both a soft and moderately hard donor, depending on metal identity—switching from preferential Cu⁺ binding in organic frameworks to Fe²⁺ coordination in biological systems.
The Geometry and Reactivity of Thiocyanate: A Lewis Perspective
Thiocyanate adopts a bent or angular geometry due to repulsions between lone pairs on nitrogen and sulfur. This distortion enhances its stereochemical flexibility, crucial in catalytic cycles and ligand substitution reactions. The bent configuration positions the lone pair donor (S and N) outwards, maximizing accessibility to transition metal centers.
Reactivity peaks when thermodynamic or kinetic favorability aligns—such as in ligand exchange processes where SCN⁻ trades coordination sites during redox or catalytic transformations. In biological contexts, this reactivity enables thiocyanate to interact with metalloenzymes, influencing electron transfer and detoxification pathways.
Industrial and Biological Significance: From Catalysts to Cellular Players
The structural insights from SCN⁻’s Lewis model directly inform its industrial applications. As a bidentate ligand, thiocyanate coordinates through S and both N atoms, forming stable complexes critical in catalytic systems such as hydrocyanation and metal-mediated cross-coupling reactions.
These complexes accelerate reaction rates and enhance selectivity, demonstrating how molecular architecture governs industrial efficiency. In biochemistry, SCN⁻’s interaction with cytochrome c oxidasase—targeting both S and N—exemplifies its dual role as a bioavailable ligand and potential inhibitor, influencing cellular redox states.
Emerging research highlights thiocyanate’s utility in material science: SCN⁻-based coordination polymers exhibit tunable porosity and conductivity, useful in gas adsorption and sensor technologies. Additionally, its (−CN)²⁻ counterpart—structurally similar but sulfur-limited—offers complementary insights, yet SCN⁻ remains the archetype for thiocyanate’s Lewis behavior due to sulfur’s pivotal donation role.
As spectroscopic and computational tools advance, the atomic-level details revealed by the Lewis structure continue to deepen understanding of ligand-metal symbiosis, reinforcing thiocyanate’s status as a cornerstone in inorganic design.
In essence, the Thiocyanate Lewis Structure is far more than a static dibyte diagram—it is a dynamic blueprint mapping electronic flow, coordination geometry, and reactivity. Its depiction unveils the molecular choreography that transforms a simple anion into a multifunctional partner in chemistry’s most demanding arenas. As researchers push boundaries in catalysis, green chemistry, and bioinorganic science, SCN⁻ stands not just as a structural curiosity but as a linchpin in the advancement of atomic-scale engineering.



Related Post

Unveiling Halle Berry's Net Worth: A Stardust Journey from Resilience to Riches

“Kiss of Life” Lyrics Unveiled: The Soul of Sade’s Timeless Anthem of Renewal

Can You Solve the Crossword Puzzle? TikToker’s Challenge Tests Memory, Vocab, and Fast Thinking

1031 Police Code Unveiled: How It Defines Property Reuse and Guides Urban Transformation