Why N₂’s Molar Mass—14.01 g/mol—Holds the Key to Modern Chemistry
Why N₂’s Molar Mass—14.01 g/mol—Holds the Key to Modern Chemistry
A single molecule of dinitrogen (N₂), with a precise molar mass of 14.01 grams per mole, underpins vast industrial, environmental, and biological processes. This molecular weight—foundational to fields ranging from steel manufacturing to climate science—defines not only N₂’s chemical behavior but also its role as Earth’s atmospheric backbone. With each nitrogen atom contributing 7 atomic mass units, their symmetrical pairing yields a stable, three-bonded diatomic molecule essential to life and industry alike.
Understanding N₂’s molar mass reveals far more than a number—it unlocks insight into molecular stability, energy efficiency, and the fabric of global化学 systems.
The Atomic Foundations of N₂’s Molar Mass
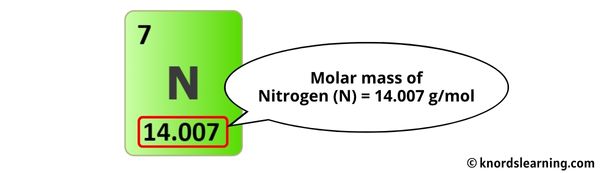
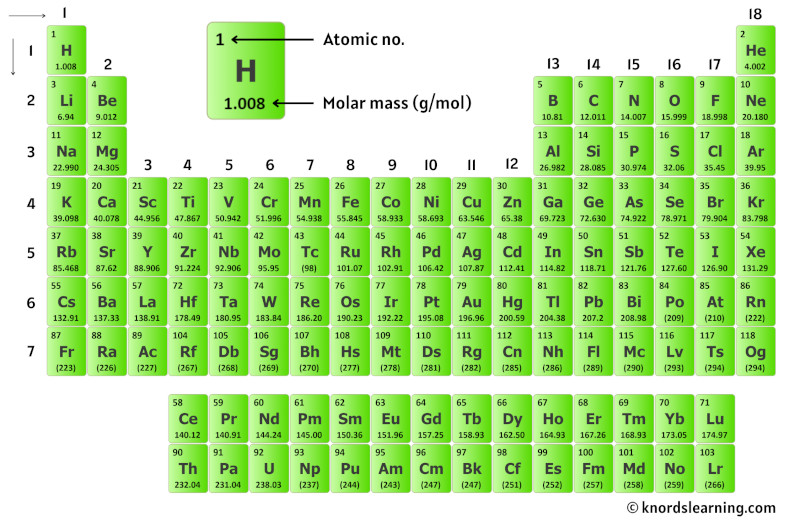
Nitrogen, element number 7 on the periodic table, possesses seven protons and seven neutrons in its most common isotopes, with electrons balancing atomic identity. The standard molar mass of N₂ (14.01 g/mol) arises from two such atoms: 7.0034 atomic mass units per nitrogen atom, totaling 14.007 g/mol when rounded.This value reflects contributions from natural isotopic abundances—primarily nitrogen-14 (99.6%) and nitrogen-15 (0.4%)—and is certified by international standards including the AVL process, which ensures ultra-pure isotopic calibration. Unlike lighter gases, N₂’s mass is significantly higher than nitrogen monoxide or hydrogen, making it a dominant yet energetically stable component of air.
The molecular structure of N₂—strong triple bond (N≡N) at ~945 kJ/mol—implies immense stability and low reactivity under ambient conditions.
This resilience explains why N₂ dominates Earth’s atmosphere, occupying 78% of dry air by volume. Its molar mass directly influences diffusion rates, solubility in liquids, and transport behaviors in biological systems. For engineers and chemists, N₂’s mass is not merely a statistical footnote—it governs reactor design, gas separation efficiency, and life-support system precision.
Industrial Demand Driven by N₂’s Molar Mass
In large-scale manufacturing, the precise molar mass of N₂ enables accurate dosing and process optimization.Consider ammonia synthesis via the Haber-Bosch process, where purified nitrogen feedstocks—typically 99.5% pure N₂—must meet strict compositional standards. A molar mass of 14.01 g/mol allows engineers to calculate exact molar flows, ensuring stoichiometric balance with hydrogen. This precision prevents catalyst poisoning and maximizes yield, with even minor deviations impacting output and energy use significantly.
Beyond ammonia, N₂’s molar mass plays a critical role in inert atmosphere processing, cryogenic storage, and semiconductor fabrication. In room-temperature applications, N₂ is preferred over air due to its non-reactivity and mass-driven inerting efficiency. For instance, in dry ice production (solid CO₂), inerting with N₂ displaces oxygen, reducing fire risk.
The 14.01 g/mol value ensures consistent performance across batches, crucial for scalable, high-purity manufacturing.
Environmental Impact and Atmospheric Significance
Earth’s atmosphere, predominantly N₂, relies on its molar mass for global biogeochemical equilibrium. The 14.01 g/mol figure ensures gravitational retention—lighter gases like hydrogen escape preferentially, while N₂ remains concentrated.This stability supports photosynthetic life: plants draw down atmospheric N₂ (via soil bacteria) and convert it into organic nitrogen, forming the foundation of terrestrial and aquatic food webs.
Climate scientists study N₂’s isotopic signatures to track anthropogenic impacts. Variations in N₂ isotopic ratios—measured relative to the standard molar mass—signal fertilizer runoff, fossil fuel emissions, or industrial nitrogen fixation.
These fingerprints reveal hidden human influence, demonstrating how a single molecular weight unit supports global environmental monitoring and policy formulation.
Biological Role and Molecular Recognition
In living systems, N₂’s role is indirect but indispensable. While most organisms cannot use atmospheric N₂ directly, enzymatic nitrogen fixation converts it into ammonia, enabling biosynthesis of proteins and nucleic acids.The molecule’s mass and triple bond architecture influence its transport across biological membranes and enzymatic recognition. For example, nitrogenase enzymes leverage N₂’s bond strength and molar mass to catalyze reduction, ensuring efficient energy transfer in microbial nitrogen scientists.
Similarly, in mammalian physiology, blood plasma contains dissolved N₂, though at trace levels.
Its molar mass affects solubility and diffusion gradients critical to respiratory gas exchange. Hemoglobin, while focused on oxygen, operates within a nitrogen-dominated environment where N₂’s stability contributes to balanced partial pressures vital for cellular respiration.
The Numerical Precision of N₂ Mass in Science and Technology
The molar mass of N₂—14.01 g/mol—is not arbitrary; it reflects meticulous measurement and international standardization.Certified by the International Union of Pure and Applied Chemistry (IUPAC), this value underpins stoichiometric calculations in academic research, industrial process design, and environmental modeling. Any deviation risks systemic errors: chemical engineers depend on it to optimize reactor yields, while climate scientists rely on it to interpret isotopic records and forecast ecosystem shifts.
In polymer synthesis, metallurgy, and pharmaceutical production, precise knowledge of N₂’s mass ensures accurate gas blending, flow control, and thermal management.
For space exploration, controlling nitrogen environments in spacecraft systems depends on the predictable behavior tied to its molecular weight. Even in everyday life—breathing air rich in inert N₂, sous-vide cooking stabilized by nitrogen purging—the molar mass quietly enables function and safety.
Takeaway: N₂’s Molar Mass—More Than a Number, a Cornerstone of Precision
From atmospheric dominance to industrial might, dinitrogen’s molar mass of 14.01 g/mol is a quiet force shaping chemistry, engineering, and life itself.Its precise value enables innovation, accuracy, and sustainability across sectors. Whether securing the integrity of a catalytic reaction or stabilizing Earth’s air, understanding N₂’s mass reveals the deep interconnection between atomic identity and global systems. This number defines not just a molecule—but the foundation of modern science and industry.
Related Post
Do Prokaryotes Have a Nucleus? The Hidden Truth Behind the Absence of a True Nucleus

Niu Football vs Fresno State Bulldogs: Analyzing Player Stats That Decided the Game’s Fate

Unlock Access to Tata Steel’s Power Core: Your Ultimate Guide to Intranet Login

Can Coca-Cola Zero Relieve Diarrhea? The Surprising Science Behind the Zero Sugar Remedy