Delta G: The Hidden Engine of Biological Energy and Cellular Survival
Delta G: The Hidden Engine of Biological Energy and Cellular Survival
In the intricate dance of life, molecular energy isn’t just a behind-the-scenes force—it’s the silent architect shaping cellular fate. At the core of biochemical efficiency and survival lies Delta G, or Gibbs free energy, a thermodynamic principle that determines whether a reaction proceeds spontaneously within living systems. By quantifying the balance between energy released and energy absorbed, Delta G dictates metabolic feasibility, drives biochemical pathways, and underpins everything from cellular respiration to cancer cell proliferation.
Understanding Delta G offers a profound lens into the energetics that sustain life at the molecular level.
The Thermodynamic Language of Life: What Delta G Really Means
Delta G, or Gibbs free energy change, measures the usable energy available to perform work in a chemical reaction at constant temperature and pressure—conditions mirroring most cellular environments. The mathematical foundation is defined by the equation ΔG = ΔH – TΔS, where ΔH represents enthalpy change, T is absolute temperature in kelvin, and ΔS is entropy change.A negative ΔG indicates a spontaneous, exergonic reaction: energy flows naturally from reactants to products, powering cellular processes. Conversely, a positive ΔG signals an endergonic reaction requiring external energy input, making it non-spontaneous without cellular intervention. In biological systems, this thermodynamic metric is not abstract—it is dynamically tuned by enzymes, buffers, and environmental conditions.
For instance, ATP hydrolysis exhibits a highly negative ΔG (approximately –30.5 kJ/mol at 25°C), making it a prime energy currency for processes like muscle contraction, active transport, and biosynthesis. This reaction releases energy by forming stable hydrolysis products, enabling cells to harness free energy for essential work. “Delta G effectively acts as the metabolic thermostat regulating what reactions can spontaneously sustain life,” explains Dr.
Elena Torres, a biophysical chemist at MIT.
Delta G Across Metabolic Pathways: From Glycolysis to Oxidative Phosphorylation
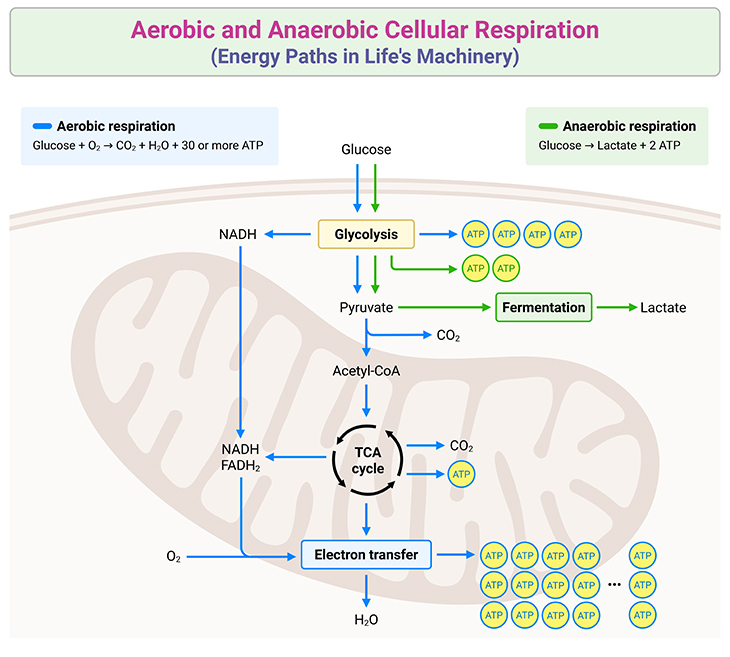
Metabolic networks operate as interconnected webs where each reaction’s Delta G influences the whole system. Glycolysis, the first major glucose breakdown pathway, features several reactions with sharply negative ΔG values, ensuring irreversible progression.The conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate, catalyzed by phosphoglycerate kinase, releases sufficient free energy to drive ATP synthesis. Meanwhile, citric acid cycle steps display nuanced ΔG profiles; for example, isocitrate dehydrogenase releases energy via a positive ΔG under physiological conditions, triggering regulation rather than spontaneous flow. Oxidative phosphorylation exemplifies Delta G’s role in energy maximization.
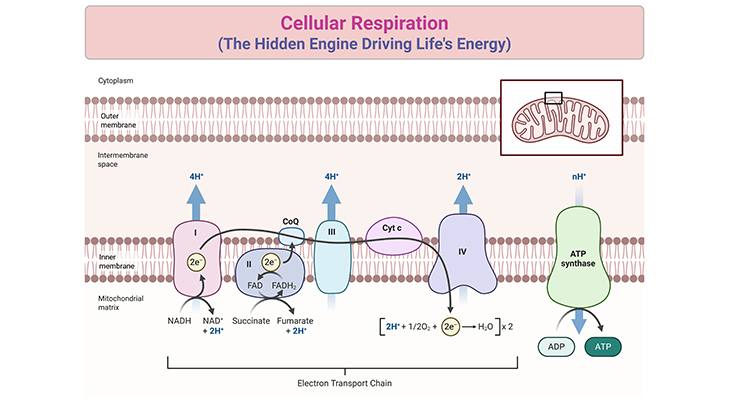
Electron transport chain proteins shuttle electrons, creating a proton gradient across the inner mitochondrial membrane. This gradient’s ΔG drives ATP synthesis through ATP synthase—a molecular turbine powering trillions of ATP molecules daily. According to professor Rajiv Mehta of Stanford University, “Delta G in these systems isn’t just a number; it’s the rationale behind every phosphate transfer and oxygen consumption.” Reactions proceed not by chance, but by design: energy-releasing steps yield downstream applications, minimizing waste and maximizing efficiency.
Subsurface Thermodynamics: How pH, Concentration, and Allostery Modulate Delta G Delta G is not static; it shifts with cellular conditions. Changes in reactant and product concentrations directly influence actual ΔG through the reaction quotient. The Nernst equation extends standard Gibbs free energy calculations to reflect physiological realities.
In acid-base balance, proton concentration alters ΔG for reactions involving proton transfer—critical in metabolic pathways like the citric acid cycle and electron transport. For example, elevated lactate levels during intense exercise skew ΔG, modulating pyruvate processing and redirecting energy flow. Allosteric regulation further fine-tunes Delta G at the molecular scale.
Key enzymes such as phosphofructokinase in glycolysis bind effector molecules, changing their shape and altering activation energy barriers. “Allostery serves as a cellular thermostat that adjusts Delta G on demand,” says Dr.


Related Post

Unlocking Cellular Precision: How Delta G Rxn Formula Drives Biochemical Equilibrium

Delta G: The Hidden Thermodynamic Force Shaping Chemical Transformations
Decoding Thermodynamics: The Critical Role of Delta G in Predicting Reaction Spontaneity
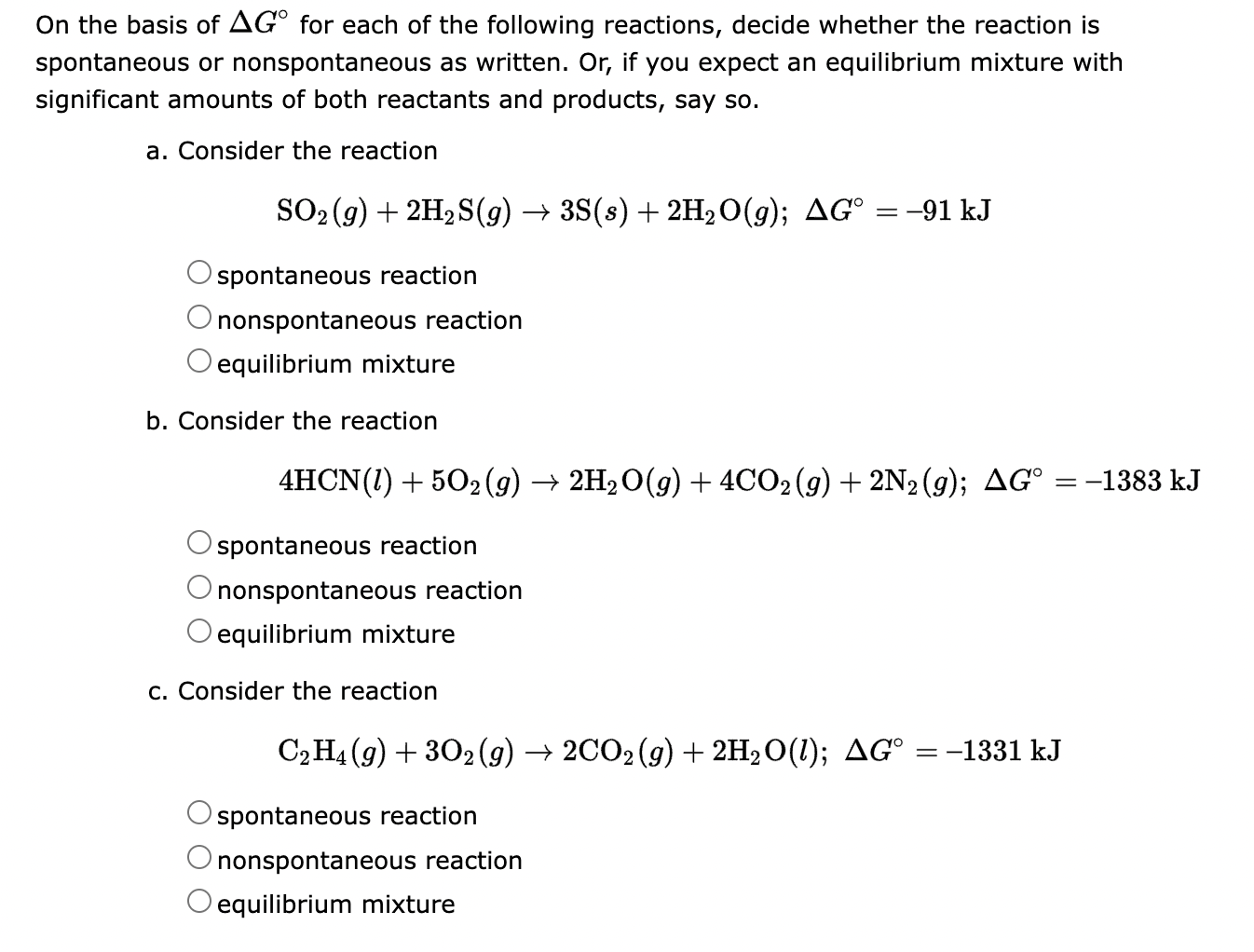
What Is Delta G? The Secret Formula Driving Spontaneous Reactions

